Abstract
The safe application of new strategies for the treatment of graft-versus-host disease (GVHD) is hampered by the lack of a clinically relevant model for preclinical testing. Current models are based on intraperitoneal transfer of human peripheral blood mononuclear cells (huPBMCs) into NOD-SCID (nonobese diabetic-severe combined immunodeficient)/SCID mice. Intravenous transfer would be preferred but this has always been ineffective. We developed a new model for xenogeneic GVHD (X-GVHD) by intravenous transfer of huPBMCs into RAG2-/- γc-/-mice. Our results show a high human T-cell chimerism of more than 20% (up to 98%) in more than 90% of mice, associated with a consistent development of XGVHD within 14 to 28 days and a total mortality rate of 85% shorter than 2 months. After murine macrophage depletion, engraftment was earlier and equally high with lower doses of huPBMCs. Human macrophages were also absent in these mice. Purified huCD3+ cells showed a similar X-GVH effect with contribution of both CD4 and CD8 phenotypes. Human immunoglobulins and cytokines were produced in diseased mice. One of 30 mice developed chronic X-GVHD with skin histology similar to human GVHD. In conclusion, we present a new model for X-GVHD by intravenous transfer of huPBMCs in RAG2-/- γc-/- mice. Murine and human macrophages do not seem to be necessary for acute X-GVHD in this model. (Blood. 2003;102:2522-2531)
Introduction
Graft-versus-host disease (GVHD) is a major complication of allogeneic bone marrow transplantation (alloBMT) and donor lymphocyte infusion (DLI). Development of new treatment modalities would be favored by the availability of a clinically relevant animal model. In such a model, intravenous transfer of human T lymphocytes should lead to the consistent development of a xenogeneic GVHD (X-GVHD), which resembles human GVHD. Human peripheral blood mononuclear cells (huPBMCs) can engraft in severe combined immunodeficient (SCID) mice, depending on the route of administration.1 Although intravenous transfer of up to 108 huPBMCs has always been ineffective, intraperitoneal transfer of huPBMCs into SCID mice has been successful.1-5 However, surprisingly little X-GVHD has been observed in these huPBMC-SCID chimeras.1,2,6-10 Failure to develop X-GVHD has been shown to be a direct consequence of a low degree of T-cell chimerism (< 10% human T lymphocytes) in most (80%) huPBMC-SCID mice.4 Low T-cell chimerism depends on the dose of huPBMCs and the conditioning regimen but the main reason is a host-versus-graft reaction by the residual innate immune system of the SCID mice (natural killer [NK] cell and macrophage activity).11-14 Several groups have addressed this problem in order to obtain a better engraftment of human cells.11-16 For example, pretreatment of SCID mice with clodronate-containing liposomes for macrophage depletion led to a prolonged circulation of huPBMCs after intravenous transfer of huPBMCs, although the engraftment rate of human cells was less than 10% and development of X-GVHD was not observed.14 In vivo NK-cell depletion in SCID mice with the use of different antibodies, directed toward NK cell-specific membrane markers (eg, anti-asialo-GM1, TM-β1, and anti-NK1.1) or NK cell products (antimurine interferon γ [IFNγ]), resulted in significantly higher engraftment rates.12,15,16 Enhanced engraftment of huPBMCs was also observed in nonobese diabetic (NOD)-SCID/SCID mice, a strain with diminished NK cell function, low complement activity, and abnormal macrophages.15,17 Although X-GVHD sometimes occurred in these mice with a T-cell chimerism of more than 10%, few groups have designed models specifically suited for the development of XGVHD. Pflumio et al18 transferred huPBMCs into newborn SCID mice, which still lack NK cell activity, and reported more than 10% human cells after 4 weeks in 40% of mice, with a subsequent development of X-GVHD in most of the mice. Sandhu et al12 developed a very successful model for X-GVHD in which SCID mice received a regimen of anti-asialo-GM1 and 3 Gy irradiation followed by 30 to 50 × 106 human peripheral blood leukocytes (huPBLs) intraperitoneally. After 2 weeks, a lethal X-GVHD developed in almost all mice and approximately 60% human T lymphocytes were found in the murine spleens. Tsuchida et al19 used the same model and found a slightly lower mortality of 70% only in the mice with more than 50% human cells in the peripheral blood (PB). However, for comparison with alloBMT and DLI settings in humans, these models have 2 disadvantages. First, although intraperitoneal transfer is successful, an intravenous transfer of huPBMCs would be preferred to better mimic the clinical situation. Second, besides their residual host resistance, NOD-SCID/SCID mice may show leakiness (restoration of functional B and T cells) and they have a tendency for spontaneous tumor formation (lymphomas, sarcomas, or thymomas).
We developed a new model for X-GVHD by intravenous transfer of huPBMCs in RAG2-/- γc-/- double-mutant mice. These mice have a more stable phenotype compared with NODSCID/SCID mice: no B, T, or NK cell activity, no leakiness, and no spontaneous tumor formation.20-22 We improved this model by depletion of murine macrophages using clodronate-containing liposomes. Our results show a high engraftment rate of human cells, with a T-cell chimerism of at least 20% in more than 90% of mice, and a consistent development of X-GVHD.
Materials and methods
Mice and conditioning regimen
RAG2-/- γc-/- mice were obtained from the Netherlands Cancer Institute (Amsterdam, The Netherlands).20 They were bred and maintained in microisolator cages under specified pathogen-free conditions at the Central Laboratory Animal Institute (Utrecht University) and received sterile water and irradiated pellets ad libitum. Female (first and second experiment) or male (third experiment) mice were used at 8 to 34 weeks of age. Mice received total body irradiation with a single dose of 350 cGy (gamma irradiation from a linear accelerator) before injection of huPBMCs on the same day. Control mice were irradiated but did not receive huPBMCs.
Preparation and transplantation of huPBMCs
Buffy coats were obtained from healthy human blood donors at the Bloodbank of the University Medical Center Utrecht. HuPBMCs were isolated by Ficoll Hypaque (Pharmacia, Uppsala, Sweden) density centrifugation and washed twice in phosphate-buffered saline (PBS). Cells were then counted and resuspended in PBS/0.1% HSA (human serum albumin) in concentrations from 5 to 30 × 106 cells/0.2 mL. Cell suspensions of 0.2 mL were injected intravenously into the irradiated mice via the tail vein. Human CD3+ cells were purified from huPBMCs by lysis of monocytes, granulocytes, and B lymphocytes using Lymphokwik solution (One Lambda, Canoga Park, CA). In order to obtain purified CD4+ or CD8+ cells, CD4+ or CD8+ cells were depleted from the CD3+ cell population using anti-huCD4 or anti-huCD8 pure antibodies (Becton Dickinson, Mountain View, CA) and goat antimouse magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany) in combination with the VarioMACS cell separation device (Miltenyi Biotech).
Preparation of clodronate-containing liposomes
Clodronate-containing liposomes were prepared as described earlier with several modifications.23 Briefly, a mixture of egg-phosphatidylcholine, egg-phosphatidylglycerol (both from Lipoid, Ludwigshafen, Germany), and cholesterol (Sigma, St Louis, MO) were dissolved in ethanol in a molar ratio of 10:1:1.5 and evaporated to dryness by rotation under reduced pressure. The lipid film was hydrated in an aqueous solution containing clodronate (Cl2MDP, concentration 60 mg/mL, Bonefos; Schering, Weesp, the Netherlands). Removal of unencapsulated clodronate was achieved by repeated washing with PBS, pH 7.4, by means of ultracentrifugation (Beckman Optima LE-80K; Palo Alto, CA) at 200 000g for 30 minutes. After the last washing step, the pellet was resuspended in PBS at a concentration of 90 mM phospholipid. Phospholipid concentration was determined according to Fiske and Subbarov as modified by King.24 The concentration of clodronate was determined spectrophotometrically at a wavelength of 238 nm after extraction and binding to Cu2+. The final clodronate concentration of the liposome formulation was within the 2 to 2.5 mg/mL range. Mice received 0.2 mL of the liposomal suspension intravenously one day before injection of the huPBMCs.
HuPBMCs, plasma, and organ collection from the huPBMC-RAG2-/- γc-/- chimeras
Mice were bled once a week under anesthesia from the retro-orbital vein. Blood was collected in EDTA (ethylenediaminetetraacetic acid)-coated cups. Erythrocytes were lysed with lysis buffer (0.17 M NH4Cl/0.1 mM EDTA/0.1% KHCO3). The cells were washed with PBS and then prepared for FACS (fluorescence-activated cell sorter) analysis. Plasma was isolated from the remaining blood and stored at -80°C for later determination of human immunoglobulins and cytokines. If mice were killed they were first anesthesized and bled from the retro-orbital vein, after which they were killed by cervical dislocation. Organs were isolated and prepared for histology and immunohistochemistry. Both femora were taken out and bone marrow (BM) was obtained for FACS analysis. Part of the spleen was used for histology and immunohistochemistry while the other part was used for FACS analysis.
FACS analysis
Single-cell suspensions from the PB, BM, and spleen were incubated for 20 minutes on ice with a mixture of appropriate fluorescently labeled monoclonal antibodies. After washing with PBS/1% fetal calf serum (FCS), 3- or 4-color fluorescent analysis of human antigens was performed on a Calibur flow cytometer (Becton Dickinson). Fractions of human cells were analyzed with Cell Quest software (Becton Dickinson). The proportion of human cells was calculated as follows: % huCD45+ = [huCD45+ (huCD45+ + mCD45+)] × 100%. The antibodies used for recognition of the specific surface molecules were mouse CD45-peridinin chlorophyll-a protein (PerCP) (pan-murine leukocytes), human CD45-allophycocyanin (APC; pan-human leukocytes), huCD3-fluorescein isothiocyanate (FITC; pan-T cells) and huCD19-phycoerythrin (PE; pan-B cells) Simultest, huCD4-FITC (T-helper cells)/huCD8-PE (T-cytotoxic cells) Simultest, huCD14-PE (pan-monocytes/macrophages), and huCD56-PE (NK cells). All antibodies were purchased from Becton Dickinson.
Histology and immunohistochemistry
Organs were stored in formaldehyde or frozen in liquid nitrogen and stored at -80°C until usage. Cryostat sections of 6 μm were put on uncoated slides and stained with hematoxylin-eosin for histology. Similar sections for immunohistochemistry were air dried, fixed in acetone, and incubated with the following antibodies: CD45-horseradish peroxidase (HRP) (DAKO, Glostrup, Denmark), huCD3-FITC, huCD4-FITC, and huCD8-FITC (Becton Dickinson). Control sections were incubated with immunoglobulin (Ig)-HRP (DAKO). Endogenous peroxidase was blocked by hydrogen peroxidase in a sodium-citrate buffer (pH 5.8) containing sodium azide. Sections for CD45 were counterstained with hematoxylin, mounted with coverslips using pertex, and analyzed microscopically. Sections for immunofluorescence were incubated with rabbit anti-FITC peroxidase (DAKO) followed by trichostatin A (TSA)-direct kit (Dupont/NEN Life Science Products, Boston, MA) for amplification of the fluorescence signal. Additionally, nuclei were counterstained with propidium iodide (Sigma-Aldrich, St Louis, MO). Subsequently, sections were analyzed by a fluorescence microscope.
Measurement of human immunoglobulins and cytokines
Total human IgM, IgG, and IgA in murine plasma were quantitated by a Beckman Coulter nephelometer (Fullerton, CA), Immage Immunochemistry System. Human granulocyte-macrophage colony-stimulating factor (GM-CSF) was measured by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (PharMingen, San Diego, CA; catalog no. 2609 KI). Other human cytokines were measured by a multiplex cytokine assay system (Bio-Plex; Bio-Rad Laboratories, Hercules, CA) with simultaneous detection of 12 human cytokines in a single sample, as described before.25 Data analysis was done with Bio-Plex Manager software (Bio-Rad Laboratories) with a 5-parametric-curve fitting.
Molecular analysis of the Vβ repertoire of human T lymphocytes
Single-cell suspensions were prepared of samples of the huPBMCs before injection in the mice and of PB, BM, and spleen samples obtained at days 2 and 16 in the third experiment. Single-step RNA isolation was performed with TRIzol Reagent (Gibco BRL, Carlsbad, CA) using manufacturer's instructions. For cDNA synthesis 6 μg of total RNA and 1 μg of a specific T-cell receptor (TCR) Cβ primer (5′-CTCCTTCCCATTCACCCACCAGCTCAGCTC-3′) were used in a 60-μL mix containing 1 × First Strand buffer, 10 mM dithiothreitol (DTT), 200 U Superscript Reverse Transcriptase (all from Gibco BRL), and 0.8 mM deoxynucleoside triphosphate (dNTP; Promega, Madison, WI), and 40 U Rnase H Inhibitor (Promega) in MilliQ water. For the polymerase chain reaction (PCR) primers, we followed the nomenclature of TCR Vβ gene families of Ferradini et al26 and Wilson et al.27 Vβ primers were used separately in combination with the GS-Cβ primer 5′-AGATCTCTGCTTCTGATGGCTC-3′.Vβ primers were described previously.28 The PCR mix contained 0.2 μL cDNA, 1 × PCR buffer (Perkin-Elmer, Roche Molecular Systems, Branch-burg, NJ), 2 mM MgCl2, 0.4 mM dNTP (Promega), 2 pmol Vβ primer, 2 pmol 5′-carboxy-fluorescein phosphoramidite-labeled GS-Cβ primer, and 0.8 U AmpliTaq DNA polymerase (Perkin-Elmer). The PCR was run under optimized conditions. Capillary electrophoresis was performed on an ABI PRISM 310 Genetic Analyzer (Perkin-Elmer). In each injection, PCR products of 2 different TCR-Vβ families with nonoverlapping fragment lengths were combined. Data were analyzed with GeneScan Analysis software and processed with Genotyper DNA fragment analysis software (Applied Biosystems, Foster City, CA).
Results
Engraftment of huPBMCs and analysis of donor variability
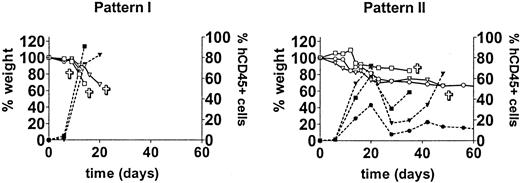
In a small-scale pilot experiment we observed a rapid engraftment of human cells after intravenous administration of huPBMCs of a single donor to irradiated mice (data not shown). In order to evaluate the reproducibility of that observation and the extent of donor variability, we repeated the experiment with cells derived from 10 different donors. Following sublethal irradiation with 350 cGy, 30 mice received 30 × 106 huPBMCs each from 10 different donors (3 mice per donor group). Three other mice were irradiated but did not receive huPBMCs and served as controls. Engraftment was monitored weekly by FACS analysis of PB. The human cells engrafted well with 9 of 10 donors. Low proportions of huCD45+ cells could already be detected at day 6 in 90% (27/30) of mice. From day 14 to 28 a huge increase in human cells, range 24% to 96%, occurred in 93% (28/30) of the mice. This increase in human cells was associated with an acute X-GVHD syndrome characterized by rapid and severe weight loss (>10%), hunched posture, ruffled fur, reduced mobility, tachypnea, and anemia. The control mice did not show any of these symptoms. Two engraftment patterns could be discriminated. These patterns can be described as early engraftment (pattern I, 5 donors) with a high mortality within 28 days, and late engraftment (pattern II, 4 donors) with a more variable mortality. Typical examples of these patterns are shown in Figure 1. Early mortality within 28 days occurred in 60% (18/30) of mice with a total mortality of 85% (26/30) within 2 months. Late engraftment usually showed a decrease in the proportion of huCD45+ cells after 4 to 6 weeks, followed by a second or third increase in human cells. These increases were associated with acute further weight loss and death of the mice. Weight loss was the most obvious sign preceding the increase in human cells and illness of the mice. The age of the mice was not related to the engraftment rate (data not shown). The increase in human cells consisted mainly of CD3+ T lymphocytes (day 20: mean, 98%; range, 86%-100%) containing both CD4 and CD8 subsets. CD4/CD8 ratios were variable between the mice as shown in Table 1. A small but consistent CD4+/CD8+ population (range, 2%-26%) could be detected in all mice. The CD4/CD8 ratio or fraction of CD4+/CD8+ T lymphocytes both seemed to be independent of the increase in huCD45+ cells or the development of X-GVHD. B cells were low to absent in PB. One mouse (9A; Table 1) survived 3 increases in T lymphocytes (at days 21, 42, and 80) and developed a chronic X-GVHD syndrome with overt hair loss, inflammation of the skin, and slow further weight loss. Of note, the fraction of CD4+/CD8+ T cells was relatively high in this mouse (12%-29%), only late in time. The mouse was killed after 3 months and immunohistochemical analysis showed human cells in all organs but especially in lungs, spleen, and skin. Skin histology showed apoptotic keratinocytes surrounded by huCD45+ cells (Figure 2) comparable with human GVHD. A lymphocytic bronchitis was seen in the lungs. The spleen showed extensive fibrosis with loss of normal structures. Focal bile duct damage was present in the liver. Immunofluorescent imaging of the skin showed infiltration of human CD3+ T lymphocytes. In conclusion, this first experiment showed an efficient engraftment by intravenous transfer of huPBMCs in RAG2-/- γc-/- mice. Almost all donors showed a high engraftment rate with the subsequent development of acute X-GVHD.
Different huPBMC engraftment patterns. The first experiment for engraftment of huPBMCs by intravenous transfer into irradiated RAG2-/- γc-/- mice was performed with 10 different donors. Engraftment occurred with 9 of 10 donors. After 2 to 4 weeks an increase in human CD45+ cells was observed (right Y-axis) and X-GVHD developed in the mice, characterized by a rapid and severe weight loss (left Y-axis). Two patterns of engraftment could be discerned: pattern I, “early” engraftment (5 donors); and pattern II, “late” engraftment (4 donors). Two groups are shown as representative examples. Symbols ▿, □, and ○ indicate 3 different mice.  indicates death of the mouse; closed symbols, % human CD45+ cells; and open symbols, weight (as a percentage of the initial weight).
indicates death of the mouse; closed symbols, % human CD45+ cells; and open symbols, weight (as a percentage of the initial weight).
Different huPBMC engraftment patterns. The first experiment for engraftment of huPBMCs by intravenous transfer into irradiated RAG2-/- γc-/- mice was performed with 10 different donors. Engraftment occurred with 9 of 10 donors. After 2 to 4 weeks an increase in human CD45+ cells was observed (right Y-axis) and X-GVHD developed in the mice, characterized by a rapid and severe weight loss (left Y-axis). Two patterns of engraftment could be discerned: pattern I, “early” engraftment (5 donors); and pattern II, “late” engraftment (4 donors). Two groups are shown as representative examples. Symbols ▿, □, and ○ indicate 3 different mice.  indicates death of the mouse; closed symbols, % human CD45+ cells; and open symbols, weight (as a percentage of the initial weight).
indicates death of the mouse; closed symbols, % human CD45+ cells; and open symbols, weight (as a percentage of the initial weight).
Skin histology of a mouse with X-GVHD compared with a control mouse and with human GVHD. Histology of the skin in a normal control mouse (hematoxylin-eosin [H&E]). Histology of the skin in a mouse suffering from X-GVHD. Arrows indicate dyskeratotic keratinocytes in the epidermis (E) and impairment of a hair follicle (H) with dyskeratotic cells accompanied by some lymphocytes (H&E). Histology of the skin in human GVHD. Arrows indicate dyskeratotic keratinocytes in the epidermis (E) hairfollicle (H) surrounded by lymphocytes (H&E). Original magnification, × 10.
Skin histology of a mouse with X-GVHD compared with a control mouse and with human GVHD. Histology of the skin in a normal control mouse (hematoxylin-eosin [H&E]). Histology of the skin in a mouse suffering from X-GVHD. Arrows indicate dyskeratotic keratinocytes in the epidermis (E) and impairment of a hair follicle (H) with dyskeratotic cells accompanied by some lymphocytes (H&E). Histology of the skin in human GVHD. Arrows indicate dyskeratotic keratinocytes in the epidermis (E) hairfollicle (H) surrounded by lymphocytes (H&E). Original magnification, × 10.
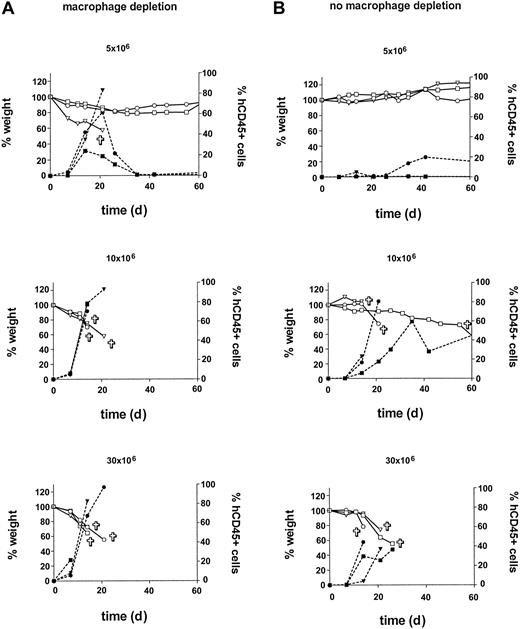
Titration of huPBMC dose and engraftment after macrophage depletion
In the second experiment, in order to further enhance engraftment, in vivo macrophage depletion was performed using clodronate-containing liposomes.29 Also, the minimum dose of huPBMCs for reproducible engraftment with a consistent development of acute X-GVHD was determined by a cell dose titration. Thirty-one mice were irradiated and huPBMCs from a random single donor were isolated. Groups A and B of 12 mice each were formed. Group A was treated with the clodronate-containing liposomes injected intravenously on day -1. Then groups A and B both received huPBMCs in a dose titration of 5, 10, 20, and 30 × 106 on day 0. Each dose was given to 3 mice. Three other mice received only sham liposomes without clodronate and served as controls for the clodronate treatment. Two other mice received only clodronate-containing liposomes, and 2 mice did not receive liposomes or huPBMCs. These mice served as controls for liposome and huPBMC treatment, respectively. Results are shown in Figure 3. In the previous experiment, an increase in human cells in the mice occurred after 14 to 28 days with the subsequent development of acute X-GVHD. In this experiment, an increase in human cells occurred again, but earlier, after 7 to 21 days in the liposome pretreated group A. This increase was also associated with acute X-GVHD in the mice, consisting of the previously mentioned symptoms with weight loss as the most obvious parameter. Control mice7 did not show any of these symptoms and gained weight in time (data not shown). Early mortality within 21 days was 83% (10/12 mice) in group A compared with 42% in group B (5/12 mice). Total mortality within 2 months was also higher in group A compared with group B (83% [10 of 12 mice] versus 58% [7 of 12 mice]). This difference in mortality between groups A and B is likely to be caused by the in vivo macrophage depletion in group A. Lower doses of huPBMCs correlated to a higher variability in engraftment and a later increase in human cells. However, later engraftment could eventually lead to the same level of human T lymphocytes in the PB and the development of an equally severe X-GVHD. The combined data from group B and the first experiment show that 30 × 106 huPBMCs is a sufficient dose for a consistent engraftment, with an early increase in human cells and development of acute X-GVHD. After liposome treatment, similar results can be obtained with a dose of 10 × 106 huPBMCs. In both groups A and B, huCD45+ cells were almost all CD3+ T lymphocytes (mean, 99%; range, 97%-100%), comparable with the first experiment. Liposome treatment did also not affect CD4/CD8 ratios and the proportion of CD4+/CD8+ T lymphocytes (Table 2). Of note, in all mice from this experiment, the T cells showed a CD4/CD8 ratio greater than 1.0 in PB initially, compared with a highly variable initial CD4/CD8 ratio between the different donor groups in the first experiment. It can also be seen that an increase in human cells from days 7 to 21 was associated with a reversal of the CD4/CD8 ratio in almost all mice. In order to further analyze the human cells that were responsible for the X-GVH effect, we injected huPBMCs and purified populations of huCD3+, huCD4+, or huCD8+ cells of the same donor after liposome treatment and irradiation in an additional 14 mice. Results showed a similar engraftment of 16 × 106 purified huCD3+ cells compared with 30 × 106 huPBMCs (containing 16 × 106 huCD3+ cells) with 100% mortality of the mice due to X-GVHD within 3 weeks in both groups. Purified populations of 13 × 106 huCD4+ or 3 × 106 huCD8+ cells of this same donor showed engraftment but with milder X-GVHD symptoms and no death of the mice. A similar X-GVH effect and mortality by purified huCD3+ cells (15 × 106) was observed for a second random donor in 6 additional mice. These data are consistent with the observation in the previous experiments of high percentages of CD3+ human T lymphocytes, consisting of both CD4+ and CD8+ cells, in the peripheral blood during the development of X-GVHD. In conclusion, this experiment showed a positive effect of in vivo macrophage depletion on the engraftment of huPBMCs in RAG2-/- γc-/- mice. Furthermore, these results indicate that human CD3+ T lymphocytes are responsible for the X-GVHD effect, with the contribution of both CD4 and CD8 T-cell phenotypes.
Influence of in vivo macrophage depletion and huPBMC dose on engraftment. Macrophage depletion with clodronate-containing liposomes was performed in group A (left). A cell-dose titration was performed in groups A and B with doses of 5, 10, 20, and 30 × 106 huPBMCs per group. An increase in human CD45+ cells (right y-axis) was associated with X-GVHD characterized by weight loss (left y-axis). Symbols ▿, □, and ○ indicate 3 different mice in each dose group.  indicates death of the mouse; closed symbols, % human CD45+ cells; and open symbols, weight (as a percentage of the initial weight).
indicates death of the mouse; closed symbols, % human CD45+ cells; and open symbols, weight (as a percentage of the initial weight).
Influence of in vivo macrophage depletion and huPBMC dose on engraftment. Macrophage depletion with clodronate-containing liposomes was performed in group A (left). A cell-dose titration was performed in groups A and B with doses of 5, 10, 20, and 30 × 106 huPBMCs per group. An increase in human CD45+ cells (right y-axis) was associated with X-GVHD characterized by weight loss (left y-axis). Symbols ▿, □, and ○ indicate 3 different mice in each dose group.  indicates death of the mouse; closed symbols, % human CD45+ cells; and open symbols, weight (as a percentage of the initial weight).
indicates death of the mouse; closed symbols, % human CD45+ cells; and open symbols, weight (as a percentage of the initial weight).
Distribution of human cells in the organs
In a third experiment, we analyzed the distribution of human cells in the organs, the production of human cytokines and immunoglobulins, and the molecular Vβ repertoire of the human T cells after engraftment. Fifteen mice were treated with clodronate-containing liposomes on day -1, followed by 350 cGy irradiation and 10 × 106 huPBMCs intravenously on day 0. Mice were killed on day 2 (group I, 5 mice), day 7 (group II, 5 mice), and day 16 (group III, 5 mice). The third group was killed as soon as weight loss became obvious and FACS analysis of PB the previous day had confirmed a high proportion of human cells. Table 3 shows the results of FACS analysis of PB, BM, and spleen. Engraftment rates of T and B cells were different for the various compartments. At all time points engraftment was highest in the spleen and lowest in the BM. Remarkably, a significant proportion of B cells was found in BM and spleen but not in the PB. In contrast, CD4/CD8 ratios were similar in PB, BM, and spleen. CD56+CD3- cells (NK cells) were very low to absent in all compartments at days 2, 7, and 16. Human CD14+ cells were absent in all compartments at days 2, 7, and 16, while the initial population (day 0) showed 32% CD14+ cells. Histology and immunohistochemistry (huCD45, huCD3, huCD4, huCD8) were performed on tongue, lungs, liver, spleen, gut, kidneys, and skin of the 15 mice that were killed on days 2, 7, and 16. Macroscopic evaluation showed splenomegaly in all mice. Hematoxylin-eosin-stained sections of the spleens showed damage to the internal structures with lymphocytic infiltrates and fibrosis (data not shown). Immunohistochemistry showed human CD45+ cells in all organs examined. Although numbers were low the first week after injection of huPBMCs, a huge increase in huCD45+ cells had occurred in all organs at day 16 comparable with the increases in human cells observed in PB, BM, and spleen. Cell numbers were highest in lungs, liver, spleen, and kidneys, while lower numbers were seen in tongue, gut, and skin (Figure 4). Immunofluorescent imaging established extensive infiltration of human CD3+ T lymphocytes in lungs, liver, and spleen. Most (65%) human lymphocytes in these organs were CD8+ (liver as an example in Figure 5) with different distribution patterns for CD4+ and CD8+ T lymphocytes. CD4+ cells were surrounding periportal areas of the liver and peribronchial areas in the lungs. CD8+ cells were more diffusely spread through the tissue. In conclusion, this third experiment showed infiltration of human CD45+ cells and CD3+, CD4+, and CD8+ T cells in all organs examined with different engraftment rates for human B and T cells in PB, BM, and spleen and a disappearance of donor CD14+ cells (macrophages) and CD56+CD3- cells (NK cells).
Infiltration of human CD45+ cells in the organs of the huPBMC-RAG2-/- γc-/- chimeras. Immunohistochemical evaluation (huCD45) was performed in 15 mice in the third experiment. Infiltration of human CD45+ cells at day 16 is shown in the various organs of the mice (right) compared with control slides (left). Original magnification, × 10.
Infiltration of human CD45+ cells in the organs of the huPBMC-RAG2-/- γc-/- chimeras. Immunohistochemical evaluation (huCD45) was performed in 15 mice in the third experiment. Infiltration of human CD45+ cells at day 16 is shown in the various organs of the mice (right) compared with control slides (left). Original magnification, × 10.
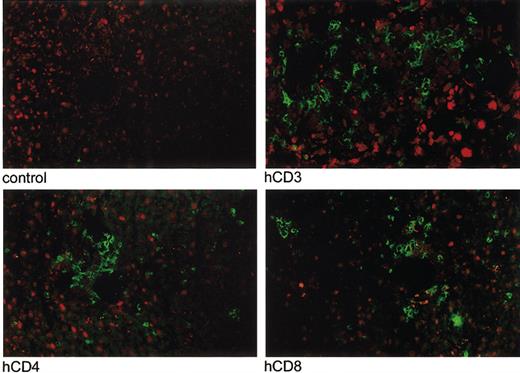
Immunofluorescent imaging of infiltration of human CD3+, CD4+, and CD8+ T lymphocytes in the liver of a mouse with acute X-GVHD. Immunofluorescent imaging was performed of the lungs, liver, and spleen of 5 mice with acute X-GVHD in the third experiment. Infiltration of human T lymphocytes, indicated by green fluorescence, was present at day 16 in all organs. An example is shown of infiltration of human CD3+, CD4+, and CD8+ T lymphocytes in the liver of one mouse compared with a control slide. Original magnification, × 10.
Immunofluorescent imaging of infiltration of human CD3+, CD4+, and CD8+ T lymphocytes in the liver of a mouse with acute X-GVHD. Immunofluorescent imaging was performed of the lungs, liver, and spleen of 5 mice with acute X-GVHD in the third experiment. Infiltration of human T lymphocytes, indicated by green fluorescence, was present at day 16 in all organs. An example is shown of infiltration of human CD3+, CD4+, and CD8+ T lymphocytes in the liver of one mouse compared with a control slide. Original magnification, × 10.
Measurement of human cytokines
In order to analyze the subtypes of human T cells involved in the development of X-GVHD, human cytokines were measured in plasma of mice from the previous experiment obtained at days 2, 7, and 16 (4 mice at each time point). Levels of human tumor necrosis factor α (TNFα) were also measured after 3 months in 1 mouse from the first experiment that had developed a chronic X-GVHD. These cytokines were chosen as they have been suggested to be involved in the development of acute human and xenogeneic GVHD.11,12,30-32 The various cytokines that were produced by the human cells in the mice are shown in Table 4. There was an intermediate production of human IFNγ, GMCSF, IL-6, IL-10, and IL-13 and a high production of IL-1α, IL-2, IL-15, and IL-18. In contrast, there was a low production of IL-4 and IL-8. Levels of human TNFα were undetectable at days 2, 7, and 16. However, human TNFα was detectable in the mouse with chronic X-GVHD from the first experiment without macrophage depletion, although the level was low (24 pg/mL). Human cytokines seemed to be produced mainly during the increase and proliferation of human cells in the mice, except IFNγ and IL-13 that were highest at day 16 when the mice had already developed a severe acute X-GVHD.
Molecular analysis of the Vβ repertoire of human T lymphocytes
Molecular analysis of the T lymphocytes in the initial huPBMC population and of the T lymphocytes that were derived from the chimeras at day 16 showed a Gaussian-like distribution in all Vβ families, indicating a polyclonal population of T cells with a normal Vβ repertoire of the T-cell receptor (Figure 6).
Molecular analysis of the Vβ repertoire of human T-lymphocyte receptors before and after engraftment. RNA was isolated from single-cell suspensions of PB, BM, and spleen as described in “Materials and methods.” Patterns of Vβ families of T lymphocytes in the initial population of huPBMCs and after engraftment in PB, BM, and spleen, measured as the relative fluorescence intensity, showed a Gaussian distribution indicative of a polyclonal repertoire of T-cell receptors. Vβ2 and Vβ22 are depicted as representative examples. Other Vβ families showed similar patterns.
Molecular analysis of the Vβ repertoire of human T-lymphocyte receptors before and after engraftment. RNA was isolated from single-cell suspensions of PB, BM, and spleen as described in “Materials and methods.” Patterns of Vβ families of T lymphocytes in the initial population of huPBMCs and after engraftment in PB, BM, and spleen, measured as the relative fluorescence intensity, showed a Gaussian distribution indicative of a polyclonal repertoire of T-cell receptors. Vβ2 and Vβ22 are depicted as representative examples. Other Vβ families showed similar patterns.
Measurement of human immunoglobulins
Human immunoglobulin levels in the huPBMC-RAG2-/- γc-/-chimeras were determined at days 2, 7, and 16. At days 2 (5 mice) and 7 (5 mice), immunoglobulin levels were undetectable. At day 16, an increase in human CD45+ cells, including B cells, had occurred and mice had developed signs of acute X-GVHD. Plasma levels in these mice5 were high for IgM and IgG (mean ± SD: IgM, 0.3 ± 0.1 mg/mL; IgG, 1.5 ± 0.8 mg/mL). Levels of IgA remained undetectable.
Discussion
In the present study we introduce a new model for X-GVHD by intravenous transfer of huPBMCs in RAG2-/- γc-/- mice. In this model we observed a high engraftment rate of human cells after 2 to 4 weeks, with a T-cell chimerism of at least 20% in more than 90% of mice. This high engraftment rate was associated with the development of acute X-GVHD and a mortality rate of 85% of the mice within 2 months. Almost all donors showed a good engraftment, although different engraftment patterns occurred. Variability of engraftment between donors is a known phenomenon in huPBMC-SCID chimeras.2,3,15,33 Analysis of immunologic parameters (phenotype markers, functional data, and human leukocyte antigen [HLA] type) has not resulted in predicting successful reconstitution in SCID mice.2 Therefore, in SCID mice it was recommended to characterize reconstitution of a specific donor each time before using their cells for experiments with large numbers of animals.2 In contrast, we have seen only one failure of 19 donors tested until now, so random donors can be used for these experiments. In vivo macrophage depletion of murine macrophages in liver and spleen using clodronate-containing liposomes showed an earlier and more consistent engraftment of huPBMCs and development of X-GVHD. The best time point for intervention in the earlier developing X-GVHD after murine macrophage depletion is currently under investigation. The disappearance of human donor CD14+ cells so soon after huPBMC injection suggests that the donor macrophages were also depleted by the liposomes. It cannot be excluded that they were absent, because of a failure to thrive in the mouse host, as literature data in huPBMC-SCID chimeras have also reported the absence of human CD14+ cells.2,7 This may also explain the absence of donor CD56+/CD3- cells (NK cells). Nevertheless, we concluded from their absence that it is unlikely for both cells to play a significant role in the development of acute X-GVHD in this model.
Histology results showed splenomegaly with fibrosis and damage of structures in the spleen. Immunohistochemistry showed infiltration of human CD45+ cells in all organs. FACS analysis showed high numbers of human cells in the spleen, whereas lower numbers were found in the BM. These observations might be characteristic for huPBMC mouse chimeras, as acute X-GVHD in huPBMC-SCID models showed similar histology results and distribution patterns of CD45+ cells.3,6,7,32 B cells are likely to reside in lymphoid compartments where they may be activated in an autonomous way or in a direct way by murine host antigens, maybe even reinforced by activated CD4+ T cells.7 This is reflected by the highly elevated plasma levels of IgG and IgM. Immunoglobulin production in huPBMC-SCID models could be high already with low numbers of B cells and was generally used as an indication of good engraftment of the human cells.1,2,10
Considering these findings, several questions remain to be answered. First, why for the first time in the development of huPBMC mouse chimeras is the intravenous instead of intraperitoneal transfer of huPBMCs effective? Several groups have tried intravenous transfer of huPBMCs without success.1,3,5 The intravenously injected huPBMCs were found in the lungs within 1 hour but could not be detected anywhere else in the mice thereafter unless extreme doses of huPBMCs were injected (up to 4 × 108).5,30 It was proposed that human accessory cells in the huPBMC were close at hand in the peritoneum for their help with the activation and proliferation of the human T cells and they would be too much dispersed throughout the mouse after intravenous transfer.7 We propose instead that the main cause of the failure of engraftment by intravenous transfer in huPBMC-NOD-SCID/SCID chimeras is the residual innate immune system of the mice (macrophages, NK cells). For huPBMC-SCID chimeras it has been shown that macrophages play a major role in the host resistance.13,14,34 In one study, engraftment by intravenous transfer of huPBMCs was successful after macrophage depletion. Although in this study T-cell chimerism was less than 10% and there were no signs of X-GVHD, this might have been due to the low number of huPBMCs injected (6 × 106).14 The earlier and more consistent engraftment of human cells after treatment with clodronate-containing liposomes is probably the result of an extra reduction in the number of residual macrophages in liver and spleen, the organs that seem most fit for initial proliferation of the human cells after intravenous injection. Furthermore, a number of studies have shown that NK cells are capable of mediating the rejection of lymphoid cells in both humans and mice.3,11,12,30,35,36 The main reason for our findings of a very high T-cell chimerism with development of X-GVHD after intravenous transfer may be the total absence of NK-cell activity in the RAG2-/- γc-/- mice. It is possible that the same results can be obtained by intravenous transfer of huPBMCs into NOD-SCID/SCID mice after NK-cell depletion. However, although various study groups achieved a better engraftment by depletion of NK cells before injection of huPBMCs, they all followed the existing successful protocols for intraperitoneal transfer.12,15,16,35 Therefore, this possibility remains to be investigated.
A second question concerns the pathogenesis of acute and chronic X-GVHD in these mice. After injection of the huPBMCs, only a small number of human cells could be detected in the PB, BM, spleen, and all other organs examined in the first week. So it seems likely, that most cells disappear. After 2 weeks, a huge increase in T lymphocytes occurred consisting of a polyclonal population with a normal Vβ repertoire that was comparable with the T lymphocytes in the initial huPBMC population. This is in contrast with the observed skewing of the Vβ repertoire of the T lymphocytes that proliferate in huPBMC-SCID chimeras, suggestive of selection of a minority of T cells that respond to murine antigens.6,7,9 These findings suggest that in our model xenospecific selection of T-cell clones does not occur. Instead, the response of the human T lymphocytes to murine antigens may be similar to a normal physiologic antigen response in humans, which is not associated with changes in T-cell-receptor spectratype. As the observed increase in human T lymphocytes consisted of both CD4+ and CD8+ cells, it seems that both subsets play a role in the development of acute X-GVHD. This was confirmed by injection of purified populations of human CD3+, CD4+, and CD8+ T cells, in which both CD4 and CD8 subsets seemed to contribute to the X-GVH effect that was obtained by injection of huCD3+ cells alone or by huPBMCs of the same donor. CD4/CD8 ratios are donor dependent and do not show a consistent pattern in time. This has also been a general observation in huPBMC-SCID models.2-4
High plasma levels of a variety of human cytokines were measured in the mice. Cytokine production was associated with proliferation and increase of human cells in the mice. Compared with data in humans, this may be a representation of the “cytokine-storm” that is an important component of acute GVHD in humans and mice.11,12,31,37 Both T-helper 1 (IL-2, IL-18, IFNγ) and T-helper 2 (IL-4, IL-10, IL-13) and inflammatory cytokines (IL-1, IL-6) seem to play a role in the development of acute X-GVHD in this model. Another important cytokine in acute human GVHD is considered to be TNFα, mainly produced by recipient human macrophages after T-helper 1 activation.37,38 The absence of human TNFα in these mice indicates that this cytokine is not important for the development of acute X-GVHD in this model. However, it may be that soluble TNFα does not reflect the whole biologic activity of this cytokine that is known to exist also in an active transmembrane form.39 In conclusion, the acute X-GVHD in the huPBMC-RAG2-/- γc-/- model may be comparable with acute human GVHD in the sense of a cytokine storm generated by activated CD4+ and CD8+ T lymphocytes. However, there are differences in the types of cytokines that are produced compared with human GVHD and we have not yet analyzed activation of host cells by interaction with human T lymphocytes.
In this set of experiments chronic X-GVHD, with skin histology comparable with human GVHD, was observed in one mouse, in which macrophages were not depleted. Plasma levels of human TNFα were detectable in this mouse, in contrast to the mice that developed acute X-GVHD. This might indicate a role for human TNFα in the development of chronic but not acute X-GVHD. There was also a remarkably high CD4+/CD8+ population in this mouse, only late in time. These cells are likely to be mature, activated T lymphocytes that can coexpress CD4 and CD8, and this observation also suggests a relationship with the development of chronic X-GVHD.6 Development of a chronic X-GVH syndrome was a general observation in recent experiments in mice that survived acute X-GVHD and contained human cells in the peripheral blood over several months (R.S.v.R., E.R.S., and S.B.E., unpublished data, February 2003). We plan to investigate the reproducibility of chronic X-GVHD and the possible differences between acute and chronic X-GVHD in these mice in future experiments.
In conclusion, we developed an easily applicable and efficient model for X-GVHD that has features comparable with human GVHD. This is the first time an intravenous instead of intraperitoneal transfer with a high engraftment rate of human T lymphocytes has been achieved in mice, which gives a better comparison with clinical alloBMT and DLI settings. Therefore, this model will be very valuable for the development of new therapies for GVHD. Furthermore, murine and human macrophages do not seem to be necessary for the development of acute X-GVHD in this model, which raises questions on the role of macrophages in human GVHD and opens up new possibilities for research in this area.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2002-10-3241.
Supported by The Netherlands Organisation for Scientific Research (NWO 920-03-199) and the Ank van Vlissingen Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Marja van Blokland for her technical assistance in Vβ analysis and Wilco de Jager for his assistance in the measurement of cytokines.

![Figure 2. Skin histology of a mouse with X-GVHD compared with a control mouse and with human GVHD. Histology of the skin in a normal control mouse (hematoxylin-eosin [H&E]). Histology of the skin in a mouse suffering from X-GVHD. Arrows indicate dyskeratotic keratinocytes in the epidermis (E) and impairment of a hair follicle (H) with dyskeratotic cells accompanied by some lymphocytes (H&E). Histology of the skin in human GVHD. Arrows indicate dyskeratotic keratinocytes in the epidermis (E) hairfollicle (H) surrounded by lymphocytes (H&E). Original magnification, × 10.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2002-10-3241/6/m_h81934989002.jpeg?Expires=1767752771&Signature=LNtmlh4YugEids89p-1WPZAAR-HBXD81Enof~qM8JOxYSyA-0ZnC7uUAm5GLgX2-uE9zFwAbJ1c-aVbqLwRiDAhl0ugE-2rkRahvT-y3q0nRTagqDPp~lO2imz7MdTB56mJOJu4WqQxKVIz3g-NvbUovjbfP2E8EVMqzN2pcaH79FlMa9coaHdVV26C1vQv8Rq4OW6x~m2BWWQ1YXNigzHcIcyOUYlnhVyjzGh~PG-Muf85RuBkY6eXScnSM9ZFZAkEe4c7bho1F4QRF2UlD9~moRsN8WZp5MXFr9OpQl8nl~AIG75U0m8wAXrHZ3CgiC36SZcrMwZ-ebsy3NGPn-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)