The association of antiphospholipid antibodies with thrombosis and obstetric events defines the antiphospholipid syndrome. A recent systematic review of the literature showed lupus anticoagulants to be risk factors of thrombosis, independent of the type and site of the event, the presence of systemic lupus erythematosus, and the laboratory methods used to detect them. Anticardiolipin antibodies were not such strong risk factors, unless arterial thrombosis, the G isotype, and medium or high titers were considered. Here, we extended the systematic review to anti–β2-glycoprotein I and antiprothrombin antibodies. Thirty-two mainly retrospective studies provided or enabled us to calculate the odds ratio (OR) with a 95% confidence interval (CI) of anti–β2-glycoprotein I and antiprothrombin antibodies for thrombosis in 5102 patients and 1973 controls. Twenty-eight studies analyzed 60 associations between anti–β2-glycoprotein I antibodies and thrombosis: 5 of 17 associations with arterial thrombosis, 12 of 21 with venous events, and 17 of 22 with any type of thrombosis were significant. Seventeen studies assessed 46 associations between antiprothrombin antibodies and thrombosis: only 17 were significant. As most studies involved patients with systemic lupus erythematosus, lupus anticoagulants, or anticardiolipin antibodies, it is difficult to establish the value of anti–β2-glycoprotein I and antiprothrombin antibodies as independent risk factors. In conclusion, the clinical significance of these antibodies still requires further investigation. However, before other clinical studies are done, standardization or at least harmonization of the methods used to detect anti–β2-glycoprotein I and antiprothrombin antibodies is mandatory.
Antiphospholipid syndrome
The combination of thromboembolic events, obstetric complications, and antiphospholipid antibodies defines the antiphospholipid syndrome.1 There are 2 forms that have been described: the “primary” syndrome,2 where there is no evidence of an underlying disease, and the “secondary” syndrome, mainly in the setting of systemic lupus erythematosus.3 In 1998, an international panel of experts met in Sapporo, Japan, to establish the classification criteria for definite antiphospholipid syndrome.1 Thrombosis may occur in any tissue or organ and, with the sole exception of superficial venous thrombosis, must be objectively confirmed by imaging or ultrasound studies or histopathology. In case of histopathologic evaluation, inflammation should not be found in the vessel wall. Obstetric events include at least one fetal death at or beyond the tenth week of gestation, or at least one premature birth at or before the thirty-fourth week, or at least 3 consecutive spontaneous abortions before the tenth week. All other causes of pregnancy morbidity must be excluded.
Antiphospholipid antibodies are a wide and heterogeneous family of immunoglobulin G (IgG) and/or IgM, or less frequently also IgA, immunoglobulins, long considered to react with negatively charged phospholipids. Lupus anticoagulants and anticardiolipin antibodies were the first 2 such antibodies to be described. Lupus anticoagulants behave as acquired inhibitors of coagulation, prolonging phospholipid-dependent coagulation,4 and anticardiolipin antibodies are usually identified by immunoassays with cardiolipin or other anionic phospholipids in solid phase.5
The “Sapporo” laboratory criteria for definite antiphospholipid syndrome are as follows: lupus anticoagulants and/or anticardiolipin antibodies must be present on 2 or more occasions at least 6 weeks apart1 ; lupus anticoagulants must be diagnosed according to the criteria proposed by the Scientific Subcommittee of Standardization of Lupus Anticoagulants/Phospholipid-dependent Antibodies6 ; anticardiolipin antibodies must be measured by a “standardized” enzyme-linked immunosorbent assay (ELISA) for β2-glycoprotein I–dependent antibodies; and IgG and/or IgM anticardiolipin antibodies have to be present at medium or high titers. According to the Sapporo definition, definite antiphospholipid syndrome is established when at least one clinical and one laboratory criteria are met.
To support the inclusion of lupus anticoagulants and anticardiolipin antibodies as laboratory criteria for the antiphospholipid syndrome in relation to thrombosis, we performed a systematic computer-assisted (MEDLINE) search of the literature published in the English language from 1988 through 2001. There were 25 prospective, ambispective, cross-sectional, and case-control studies on more than 7000 patients and controls that provided or enabled us to calculate the OR with a 95% CI of lupus anticoagulants and anticardiolipin antibodies for arterial and/or venous thrombosis (Table 1).7 The review formally established that lupus anticoagulants are strong risk factors for thrombosis, regardless of the site (arterial or venous) and type (first event or recurrence), the presence of systemic lupus erythematosus, and the coagulation tests used to detect them. Anticardiolipin antibodies were not such strong risk factors, and less than half of their associations with thrombosis (16 of 33) were significant, unless the G isotype and medium or high titers were considered. Separate analysis of the different types of thrombosis showed anticardiolipin antibodies were associated with cerebral stroke and myocardial infarction, but not with deep vein thrombosis.
There were 5 studies that directly compared lupus anticoagulants and anticardiolipin antibodies for their OR for thrombosis in a few hundred patients and controls: the former, but not the latter, antibodies were significantly associated with thrombosis. Although indirect and potentially risky, comparison of the studies that analyzed only one antibody confirmed the increasing awareness that lupus anticoagulants are better predictors of thrombosis than anticardiolipin antibodies.
In conclusion, this systematic review confirms the inclusion of lupus anticoagulants as laboratory criterion of the antiphospholipid syndrome in relation to arterial and venous thrombosis. Uncertainty still exists with respect to anticardiolipin antibodies.
Anti–β2-glycoprotein I and antiprothrombin antibodies: new candidates in the antiphospholipid syndrome
Work done in the 1990s made it clear that the true antigenic targets of antiphospholipid antibodies are not the phospholipids per se, but plasma proteins bound to an anionic (not necessarily phospholipid) surface. Among them, β2-glycoprotein I,8-10 prothrombin,11-13 (activated) protein C,14 protein S,14 annexin V,15 high- and low-molecular-weight kininogens,16 oxidized low-density lipoproteins,17 tissue plasminogen activator,18 coagulation factor XII,19 complement component C4,20 complement factor H,20 and coagulation factor VII/VIIa21 have been described. As most of these proteins are involved in the regulation of blood coagulation, antibodies that lower their plasma concentration or hamper their function may cause an imbalance between the pro- and anticoagulant systems. This might explain, at least partly, the increased risk of thrombosis in antiphospholipid-positive patients. Antibodies to β2-glycoprotein I and prothrombin are the 2 most frequent and best-studied antiphospholipid antibodies. A number of excellent reviews on their immunologic and functional properties have been published.22-25
Since their first description, the relationship between anti–β2-glycoprotein I and antiprothrombin antibodies and thrombosis has been amply studied. Investigators are now inclined to consider them good candidate laboratory criteria for the antiphospholipid syndrome, together with or possibly in place of lupus anticoagulants and anticardiolipin antibodies.
To contribute to this issue, we extended our MEDLINE search of the literature to studies on anti–β2-glycoprotein I and antiprothrombin antibodies. All series of 10 or more patients were classified according to the antiphospholipid antibody type and underlying disease, and information about study design and assay methods was recorded. No attempt was made to select them on the basis of laboratory methods. Odds ratio with 95% CI of the antibodies for arterial and/or venous thrombosis were recorded or, if not available, calculated for each study, using contingency tables. An association was considered statistically significant when the lower limit of the 95% CI was higher than 1.0.
It was virtually impossible to select studies on the basis of their design, as most were retrospective: the lack of objective documentation of thrombosis, of temporal sequence between measurement of the antibodies and the time of the events, and of a control group greatly reduces their level of evidence. For only a few studies could we formally establish the strength of the association. The cross-sectional and case-control design is a potential limitation in that it is based on the assumption that the level and type of antibodies measured at or after the event reflect the antibody status before the event. This is not necessarily true, unless the antibodies are measured very shortly after the event. Furthermore, the objective documentation of (previous) thrombosis in cases and the exclusion of thrombosis in controls are crucial in cross-sectional and case-control studies, as they can influence the correct classification of patients. Indeed, in the former studies the control group comprises patients in whom the clinical event has been ruled out by instrumental evaluation.
There were 37 articles retrieved on anti–β2-glycoprotein I or antiprothrombin antibodies for this systematic review.13,26-61 In 5 studies57-61 the OR with 95% CI for thrombosis was neither provided nor could it be calculated, and these studies were therefore excluded. The main characteristics of the remaining 32 articles are reported in Table 2.
Cross-sectional and case-control studies gave information on 1324 patients and 1973 controls, and retrospective studies contributed another 3778 patients.
Systemic lupus erythematosus, the antiphospholipid syndrome, and the presence of lupus anticoagulants and/or anticardiolipin antibodies were the enrolment criteria in 26 studies. This makes it difficult to establish the relative roles of anti–β2-glycoprotein I and antiprothrombin antibodies as independent risk factors of thrombosis. Only 11 studies did multivariate analysis using logistic regression, which allows a summary of the risk assessment given the joint contribution of each risk factor.
Combining the results of relevant studies in a meta-analysis is the currently preferred method for summarizing information from the literature. Unfortunately, various biases can threaten the results of a meta-analysis,62 so that pooling data is not always appropriate, even when dealing with well-defined randomized clinical trials. Meta-analysis of observational studies is even more problematic, and needs special caution.63,64 In the case of antiphospholipid antibodies, we found too many sources of heterogeneity to allow any meaningful data pooling. We also avoided rating the quality of studies in order to conduct the meta-analysis in a subset. This practice has been criticized even for clinical trials65 and anyway no validated scales are available for observational studies of risk factors. Thus, our evaluations are based on the number of significant results supporting an association. This approach would certainly be misleading, particularly if the studies on each type of thrombosis differed in their power to provide significant results. This eventuality was judged unlikely, in view of the similarity of the 95% CI widths of the OR.
For analysis, studies were grouped according to the antiphospholipid antibody. Anti–β2-glycoprotein I antibodies were also compared, whenever possible, with anticardiolipin antibodies for their association with thrombosis.
Studies on anti–β2-glycoprotein I antibodies
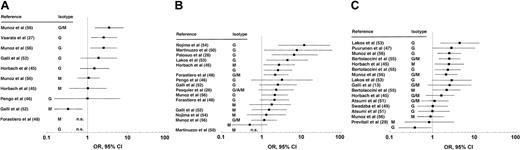
The OR with 95% CI of anti–β2-glycoprotein I antibodies for thrombosis were available or could be calculated in 28 studies on 4394 patients and 1973 controls (Table 2 and Figure 1). One study30 used 2 methods to detect the antibodies: (1) using β2-glycoprotein I in solid phase, and (2) measuring β2-glycoprotein I–dependent anticardiolipin antibodies. We report the results of the first assay. The results with the latter test are shown in “Comparison of anti–β2-glycoprotein I with anticardiolipin antibodies.”
Anti–β2-glycoprotein I antibodies and thrombosis: OR with 95% CI grouped according to the type of thrombosis. (A) Arterial thrombosis. (B) Venous thrombosis. (C) Any thrombosis. n.s. indicates not significant.
Anti–β2-glycoprotein I antibodies and thrombosis: OR with 95% CI grouped according to the type of thrombosis. (A) Arterial thrombosis. (B) Venous thrombosis. (C) Any thrombosis. n.s. indicates not significant.
Overall, 34 (57%) of 60 associations reached significance: 5 of 17 associations with arterial thrombosis, 12 of 21 with venous thrombosis, and 17 of 22 with any thrombosis (when no distinction was possible between venous and arterial thrombosis). Analysis in relation to the isotype showed IgG anti–β2-glycoprotein I antibodies were significantly associated with thrombosis in 20 (61%) of 33 cases, IgM in 7 (47%) of 15 cases, IgA in 3 (100%) of 3 cases, and the G/A/M isotypes (when no distinction was possible) in 4 (44%) of 9 cases. Analysis in relation to the presence of systemic lupus erythematosus (or, less commonly, other autoimmune diseases) showed that 24 (71%) of 34 and 10 (38%) of 26 associations with thrombosis were significant in patients with and without the disease. There were 10 studies that included multivariate analysis: 2 of them40,48 confirmed that IgG anti–β2-glycoprotein I antibodies were independent risk factors for venous thrombosis.
Anti–β2-glycoprotein I isotypes and titers are 2 important issues. The former could be tackled only partly by our review, as most studies investigated only IgG antibodies, or did not distinguish between isotypes. Overall, IgG anti–β2-glycoprotein I antibodies seemed more consistently associated with thrombosis than IgM antibodies. IgA anti–β2-glycoprotein I antibodies were always significantly associated with thrombosis. However, as only one study was available, no definite conclusion can be reached about the clinical significance of this isotype. The lack of reference materials to quantify anti–β2-glycoprotein I antibodies meant we could not assess whether the risk correlated with their titers.
Although these data suggest that IgG anti–β2-glycoprotein I antibodies are associated with thrombosis, a number of issues raise concern. First, significant associations were reported only by retrospective studies, which have a low level of evidence. Second, only a minority of studies confirmed these findings by multivariate analysis. Finally, when the antibodies were analyzed in relation to the type of thrombosis, they were not associated with arterial events, and only marginally with venous events. In conclusion, therefore, the role of anti–β2-glycoprotein I antibodies as laboratory criteria for the antiphospholipid syndrome remains to be established.
Comparison of anti–β2-glycoprotein I and anticardiolipin antibodies
There were 20 studies that compared anti–β2-glycoprotein I with anticardiolipin antibodies for their association with arterial and/or venous thrombosis.25,27,29,31,35-44,46-51 Overall, 20 associations reached statistical significance in the case of anti–β2-glycoprotein I antibodies, and 16 were significant for anticardiolipin antibodies (Table 3). Analysis in relation to the type of thrombosis showed that anticardiolipin antibodies seemd more often associated with arterial events, and anti–β2-glycoprotein I antibodies with venous thrombosis. This confirms our previous review,7 which also found that anticardiolipin antibodies were associated with arterial and not venous thrombosis.
The comparison of our systematic reviews showed other differences between anti–β2-glycoprotein I and anticardiolipin antibodies. Systemic lupus erythematosus apparently did not influence the risk of thrombosis borne by anticardiolipin antibodies.7 In contrast, the number of significant associations between anti–β2-glycoprotein I antibodies and thrombosis was particularly high in patients suffering from this autoimmune disease. We have no convincing explanation for these differences, which might be related to the design of the studies used for the 2 reviews.
The differences in the associations with thrombosis of anti–β2-glycoprotein I and anticardiolipin antibodies are surprising, taking into account that most anticardiolipin antibodies recognize β2-glycoprotein I bound to cardiolipin and other negatively charged phospholipids in solid-phase assays.8-10 They are not completely identical, because β2-glycoprotein I–independent anticardiolipin antibodies do exist, and are commonly found in conditions not involving thrombosis.66-68 ELISA for the direct measurement of anti–β2-glycoprotein I antibodies avoids their detection. This is thought to increase the assay specificity toward the clinical manifestations of the antiphospholipid syndrome. The results of our systematic review only partly support this.
Analysis in relation to the antibody isotypes did not show any difference between anti–β2-glycoprotein I and anticardiolipin antibodies for their association with thrombosis (Table 4).
Studies on antiprothrombin antibodies
The OR with 95% CI of antiprothrombin antibodies for thrombosis were available or could be calculated in 17 studies on 2339 patients and 613 controls (Table 2; Figure 2). In one study54 antiprothrombin antibodies were detected by 2 methods, utilizing human prothrombin either directly coated on the plate, or complexed with phosphatidylserine. Here, we considered the results with the latter ELISA, which gave the highest correlations with thrombosis.
Antiprothrombin antibodies and thrombosis: OR with 95% CI grouped according to the type of thrombosis. (A) Arterial thrombosis. (B) Venous thrombosis. (C) Any thrombosis. n.s. indicates not significant.
Antiprothrombin antibodies and thrombosis: OR with 95% CI grouped according to the type of thrombosis. (A) Arterial thrombosis. (B) Venous thrombosis. (C) Any thrombosis. n.s. indicates not significant.
Overall, 17 (37%) of 46 associations were significant: 3 of 11 associations with arterial thrombosis, 7 of 18 with venous thrombosis, and 7 of 17 with any thrombosis reached significance. Analysis in relation to the isotype showed IgG antiprothrombin antibodies were associated with thrombosis in 11 (26%) of 24 cases, the M isotype in 2 (14%) of 14 cases, and the G/A/M isotypes in 4 (50%) of 8 cases (Figure 2). Analysis in relation to the presence of systemic lupus erythematosus showed 8 (53%) of 15 and 9 (29%) of 31 associations with thrombosis were significant in patients with and without the disease. There were 8 studies that did a multivariate analysis: 2 of them28,54 confirmed that antiprothrombin antibodies were independent risk factors for thrombosis and 3 others showed that they added to the risk borne by lupus anticoagulants51,56 or anticardiolipin antibodies.27
In conclusion, no clear association with thrombosis was found for antiprothrombin antibodies, irrespective of isotype, site and type of event, and systemic lupus erythematosus. Whether their presence further increases the risk of thrombosis carried by lupus anticoagulants and anticardiolipin antibodies has still to be defined. Also, the utility of their detection in clinical practice remains to be established.
Anti–β2-glycoprotein I and antiprothrombin antibodies: open problems in laboratory detection
Antiphospholipid antibodies are essential criteria for the diagnosis of the antiphospholipid syndrome, so their correct identification is vital. Laboratory diagnosis of anti–β2-glycoprotein I and antiprothrombin antibodies mostly uses “homemade” ELISAs, whose main weakness is the lack of appropriate standardization. The European Forum on Antiphospholipid Antibodies made an international survey of anti–β2-glycoprotein I measurement. The results were disappointing, as the prevalence of concordant results was 37% for IgG and 27% for IgM antibodies among 21 centers.69 However, it must be noted that most samples were in the low positivity range, and that agreement was more satisfactory on anti–β2-glycoprotein I results with high- and medium-positive samples.
So far, no survey has been done for antiprothrombin antibodies. Donohoe et al61 attempted to find the optimal assay conditions for the detection of antiprothrombin antibodies, identifying a number of preanalytical and analytical factors that may influence the test outcome. In general, heterogeneity in reagents, calibrators, assay conditions, and how the results were calculated are the main variables responsible for discrepancies in ELISAs for both anti–β2-glycoprotein I and antiprothrombin antibodies.
Several clinical studies included in our systematic review are 5 to 10 years old. Thus, the unsatisfactory results we show may depend on the use of assays that are not in keeping with today's methods. It is also possible that the use of commercial kits may make it easier to establish an association between the risk of thrombosis and the presence of antiphospholipid antibodies. There are 4 studies included in this review that used commercially available ELISAs to measure anti–β2-glycoprotein I antibodies: 29,40-42 only one41 showed these antibodies were associated with thrombosis. None of the studies analyzed here used commercially available ELISAs for the detection of antiprothrombin antibodies. Thus, it is not clear whether commercial kits offer an advantage over homemade assays.
In conclusion, the ELISAs currently used to detect anti–β2-glycoprotein I and antiprothrombin antibodies are far from properly standardized. The development of common protocol procedures and the production of reference materials are underway and should—it is hoped—help improve harmonization among laboratories.
Conclusions and future prospects
During the last decade dozens of studies enrolling several thousands of patients have investigated the clinical significance of antiphospholipid antibodies. We reviewed a wide selection of these studies, and formed the opinion that they are, at best, inconclusive. Only lupus anticoagulants were consistently associated with thrombosis, which implies that measuring them is helpful to define the patients' risk of arterial and venous thrombosis, and to guide therapeutic management. The results of studies on anticardiolipin and anti–β2-glycoprotein I antibodies are less convincing and partly controversial, but they leave open the possibility that their measurement too may be practical and useful, at least in some situations. At present, there does not seem to be any role for measurement of antiprothrombin antibodies. Although appealing, scoring the clinical relevance of the various antiphospholipid antibodies is premature and probably meaningless. A number of issues remain to be clarified.
Firstly, as anti–β2-glycoprotein I and anticardiolipin antibodies are closely related, and often identical, they could be expected to be associated with the same clinical events of the antiphospholipid syndrome. However, our reviews found the former were preferentially associated with venous thrombosis and the latter with arterial events.
Next, anti–β2-glycoprotein I and antiprothrombin antibodies both have lupus anticoagulant effect. Thus, one might expect that ELISA assays, specifically measuring each single antibody, should offer an advantage over clotting tests, which give only a qualitative estimate of an in vitro phenomenon, or that at least they are comparable in their correlations with clinical outcomes. However, our 2 systematic reviews do not provide support for the still-debated decision about whether to replace coagulation tests with ELISAs.
Finally, although their effect has been investigated little in vitro and in animal models of thrombosis, antiprothrombin antibodies may have a role in the development of the clinical manifestations of the antiphospholipid syndrome. Nevertheless, our systematic review did not find an association between antiprothrombin antibodies and thrombosis.
Taking into account their limitations, our reviews of the antiphospholipid syndrome do not reach any firm conclusions, but are aimed more at appraising the combined evidence, in order to retrieve the maximum amount of certainty from the information and guide future research in this field. We foresee the need for well-designed clinical trials, to help establish which, if any, among the various antibodies, are risk factors for the antiphospholipid syndrome. To accomplish this, standardization or at least harmonization of the methods used to detect the antiphospholipid antibodies is mandatory. Without it, any clinical study will be criticizable and unable to reach firm conclusions.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2002-11-3334.
We thank J. Baggott for kindly revising the English in our manuscript.