Abstract
Chemokines are thought to control lymphocyte recruitment to the inflamed endothelium. To dissect chemokine-mediated adhesion, binding of ex vivo isolated splenocytes to tumor necrosis factor (TNF)–activated endothelial cells was analyzed under shear stress. We observed specific adhesion of naive follicular B cells, which could be blocked by pertussis toxin. This indicated a G protein–mediated binding and pointed at a contribution of chemokine receptors to B-cell adhesion. Analysis of chemokines expressed by TNF-activated endothelial cells showed that CC chemokine ligand 2 (CCL2), CCL17, and CCL20 were up-regulated. Only on follicular B cells was the cognate receptor for CCL20, CC chemokine receptor 6 (CCR6), expressed strongly, and a functional transmigration assay with CCR6-negative B cells demonstrated conclusively the sole signaling of CCL20 through CCR6. Desensitization of CCR6 on naive B cells with CCL20 resulted in receptor down-regulation and reduced B-cell adhesion. We conclude that CCL20 plays a vital role in B-cell adhesion to the inflamed endothelium.
Introduction
The movement of cells through the blood vessel wall is a highly regulated multistep process.1,2 The first step, the adhesion of lymphocytes, requires an activation of integrins mediated by chemokines.3 These activation signals can be blocked with pertussis toxin (Ptx)4,5 and have been shown to be transferred via Gαi-coupled chemokine receptors.6 The luminal exposition of the chemokines CC chemokine ligand 19 (CCL19) and CCL21 has recently been described for the lymph node vasculature and has been shown to be important for lymphocyte recirculation.7,8 In contrast, the contribution of chemokines to cell migration into sites of inflammation remains ill-defined for most tissues.9
We tested the ability of naive nonactivated splenocytes to adhere to tumor necrosis factor (TNF)–activated bEnd.3 mouse endothelial cells. Only naive follicular B cells bound to the endothelial cell layer in a TNF-dependent and Ptx-sensitive manner. To address the role of endothelial cell–produced chemokines for B lymphocyte adhesion in inflammatory situations, we tested the competence of bEnd.3 endothelial cells to produce chemokines after activation with the proinflammatory cytokine TNF. Of 15 chemokines analyzed, CCL2, CCL17, and CCL20 mRNAs were found to be expressed. Furthermore, we could show that the presence of endothelial CCL20 protein correlates with the adhesion of naive B cells in vitro.
Study design
Mice and cells
As sources of CCR6-positive and CCR6-negative lymphocytes, spleens from C57BL/6 and C57BL/6–CCR6-negative mice10 were harvested, and lymphocytes were isolated for further use.
Antibodies and recombinant proteins
The following antibodies were used for flow cytometry: rat antimouse T-cell receptor β (TCRβ) chain (fluorescein isothiocyanate [FITC]–conjugated; BD PharMingen, Heidelberg, Germany), rat antimouse CD45R/B220 (peridinin chlorophyll protein [PerCP]–conjugated; BD PharMingen), rat antimouse CD21/CD35 (FITC-conjugated; BD PharMingen), rat antimouse CD23 (phycoerythrin [PE]–conjugated, BD PharMingen), and rat antimouse CCR6 (R&D Systems, Wiesbaden, Germany). For activation of the endothelial cells, murine recombinant TNF (BD PharMingen) was used. Lymphocyte adhesion was inhibited with Ptx (List Laboratories, Campbell, CA). Recombinant chemokines were purchased from R&D Systems.
Flow cytometry and cell sorting
Multicolor flow cytometry was performed and analyzed as described.13 The total number of cells per sample was quantified using 1 × 104 beads per sample. Inbred C57BL/6 (B6.WT) mice were purchased from Charles River (Sulzfeld, Germany). Mice with an age of 8 to 16 weeks were used. Splenic B-cell subpopulations for RNA extraction were labeled with monoclonal antibodies (mAbs) and separated with a MoFlo cell sorter (Cytomation Bioinstruments, Freiburg, Germany). Alternatively, the AutoMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany) was employed using anti-CD43 MicroBeads (“untouched B cells,” Miltenyi Biotec).
RNA extraction and reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was extracted from cells using the Perfect RNA mini kit (Eppendorf, Hamburg, Germany) according to the manufacturer's instructions. First-strand cDNA was synthesized from 1 to 2 μg total RNA. The iCycler IQ-PCR System (Bio-Rad Laboratories, München, Germany) was used to quantify gene expression with SYBR-Green (Roche Applied Biosciences, Mannheim, Germany) following the manufacturer's instructions. The following primers were used at a concentration of 200 nM: CCR2 (sense: 5′-GAGCCTGATCCTGCCTCTACTTGT-3′, antisense: 5′-CCTGCATGGCCTGGTCTAAGTGC-3′), CCR4 (sense: 5′-AAGGCATTTGGGGAGGTCTTCC-3′, antisense: 5′-AAGCCCACCAGGTACATCCATG-3′), CCR6 (sense: 5′-CATACTCTTTGTCCTCACCCTACC-3′, antisense: 5′-GCTTGAGATG ATGATGGAGATGAA-3′), CCR8 (sense: 5′-CGGGGCTGCCTGAACCAC-3′, antisense: 5′-CCCACGGGCATTTGTCTCC-3′), CXCR4 (sense: 5′-GGTACATCTGTGACCGCCTT-3′, antisense: 5′-GGCGAGGGCCTCTGTGATGG-3′), CCL20 (sense: 5′-TGCGGTGGCAAGCGTCTG-3′, antisense: 5′-CCCAGCTGTGATCAT TTCCTCCTT-3′), CCL17 (sense: 5′-CAGGAAGTTGGTGAGCTGGTATA-3′, antisense: 5′-TTGTGTTCGCCTGTAGTGCATA-3′), CCL2 (sense: 5′-CCAGCTCTCTCTTCCTCC-3′, antisense: 5′-GGCATCACAGTCCGAGTC-3′), and β-actin.14
Chemokine-specific ELISAs
Chemokine-specific enzyme-linked immunosorbent assays (ELISAs) were performed to detect CCL2, CCL17, and CCL20 in the supernatant of endothelial cells. CCL2 and CCL17 were investigated with DUO-ELISA kits (R&D Systems) following the manufacturer's instructions.
For the CCL20, ELISA plates were coated with a goat antimouse CCL20 polyclonal immunoglobulin (Ig) as capture antibody (R&D Systems). To detect the chemokine a rabbit anti-CCL20 peptide serum (sequence: KRAVNNLSLRVKKM) was generated. Recombinant mouse CCL20 (R&D Systems) was used as a standard.
Adhesion and transmigration assay
An adhesion assay with lymphocytes on bEnd.3 monolayers was performed under shear stress as described.11,15 Splenoncytes were incubated with or without Ptx (100 ng/mL; List Laboratories) in RPMI (10% fetal calf serum [FCS]; PAN Biotech, Aidenbach, Germany), harvested, and washed once. Lymphocytes were placed on the endothelial cell layer at a density of 1 × 106 cells per 100 μL per well. Cells were allowed to adhere for 20 or 30 minutes at room temperature under shear on an orbital shaker at 75 rounds per minute. The lymphocyte adhesion assay was analyzed and quantified by flow cytometry.
To test the ability of lymphocytes to transmigrate in response to CCL20 and CXCL12, CCR6-positive and -negative lymphocytes were used in transwell chambers (Corning, Bodenheim, Germany). B cells (2 × 105 “untouched” B cells) were added to the upper chamber, chemokines at the concentrations indicated to the lower chamber.
To desensitize CCR6 on B cells,16 splenocytes were incubated for 10 minutes prior to the transmigration or the adhesion assay with 100 ng or 10 and 1000 ng/mL recombinant murine CCL20 (R&D Systems), respectively.
Results and discussion
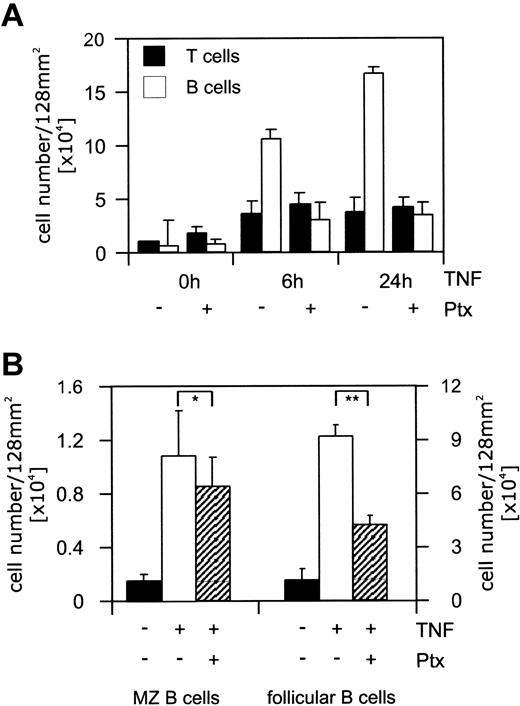
T and B cells were isolated by magnetic-activated cell sorting (MACS), and 2 million cells of either lymphocyte population were added to the TNF-activated endothelial cells. Of B or T cells, 9% or 2%, respectively, adhered under shear stress (Figure 1A). This constituted a 10-fold increase in B-cell adhesion to TNF-activated endothelial cells (Figure 1A). The pretreatment of lymphocytes with Ptx interfered with the adherence of splenic B cells and reduced their binding to 20% of the maximal adhesion (Figure 1A). Adhesion of splenic T cells was only marginally increased by activation of endothelial cells (2-fold) and was not sensitive to Ptx treatment (Figure 1A). To further dissect the striking adhesion of B cells, adherent B lymphocytes were electronically subdivided into follicular and marginal zone (MZ) B cells according to the expression levels of CD21 and CD23.17 The interaction of both B-cell populations with activated endothelial cells resulted in a comparable adhesion. However, treatment with Ptx inhibited the adherence of follicular B cells but not of MZ B cells (Figure 1B).
Specific adhesion of follicular B cells to activated endothelial cells. Endothelial bEnd.3 cells were stimulated with recombinant TNF (5 ng/mL) for the times indicated. To inhibit chemokine receptor-mediated signals lymphocytes were incubated with Ptx. (A) T (▪) or B cells (□) were separated, placed on the endothelial cell layer at a density of 1 × 106 cells per well (64 mm2), and allowed to adhere for 30 minutes at room temperature under shear stress. Adherent cells were harvested by incubating the wells with PBS/EDTA (phosphate-buffered saline/ethylenediaminetetraacetic acid) (5 mM). Two wells were pooled (128 mm2), flow cytometrically analyzed, and quantified. The total cell number of adherent B or T cells is presented. (B) Lymphocytes were placed on the endothelial cell layer (stimulated with recombinant TNF for 24 hours) at a density of 1 × 106 cells per well (64 mm2). The adhesion assay was performed as described. ▪ indicates nonactivated endothelial cells; □, TNF-activated endothelial cells; and ▨, TNF-activated endothelial cells plus Ptxtreated B cells. Recovered cells were electronically gated, and MZ (CD21+ CD23–)or naive follicular B cells (CD21+ CD23+) were quantified. Only the inhibition of CD21+ CD23+ naive B-cell adhesion by Ptx was significant (*P > .05, **P ≤ .001). Results are presented as mean ± SEM of 2 combined wells. Statistical significance was determined using an unpaired Student t test.
Specific adhesion of follicular B cells to activated endothelial cells. Endothelial bEnd.3 cells were stimulated with recombinant TNF (5 ng/mL) for the times indicated. To inhibit chemokine receptor-mediated signals lymphocytes were incubated with Ptx. (A) T (▪) or B cells (□) were separated, placed on the endothelial cell layer at a density of 1 × 106 cells per well (64 mm2), and allowed to adhere for 30 minutes at room temperature under shear stress. Adherent cells were harvested by incubating the wells with PBS/EDTA (phosphate-buffered saline/ethylenediaminetetraacetic acid) (5 mM). Two wells were pooled (128 mm2), flow cytometrically analyzed, and quantified. The total cell number of adherent B or T cells is presented. (B) Lymphocytes were placed on the endothelial cell layer (stimulated with recombinant TNF for 24 hours) at a density of 1 × 106 cells per well (64 mm2). The adhesion assay was performed as described. ▪ indicates nonactivated endothelial cells; □, TNF-activated endothelial cells; and ▨, TNF-activated endothelial cells plus Ptxtreated B cells. Recovered cells were electronically gated, and MZ (CD21+ CD23–)or naive follicular B cells (CD21+ CD23+) were quantified. Only the inhibition of CD21+ CD23+ naive B-cell adhesion by Ptx was significant (*P > .05, **P ≤ .001). Results are presented as mean ± SEM of 2 combined wells. Statistical significance was determined using an unpaired Student t test.
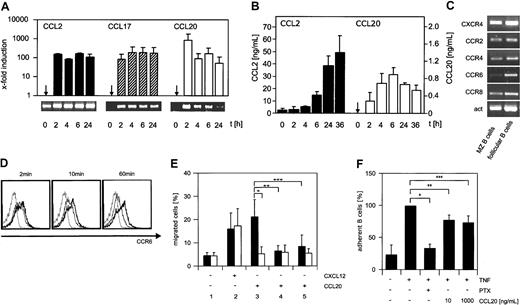
In a screen for chemokines expressed by TNF-activated endothelial cells, CCL2 mRNA was expressed constitutively in nonactivated cells and strongly up-regulated during activation (Figure 2A, lower panel). In contrast, CCL17 and CCL20 mRNA were not present in nonactivated endothelial cells but could only be detected after activation (Figure 2A). Neither CCL21, CXCL12, nor CXCL13, 3 prominent lymphocyte-attracting chemokines, were detectable. CCL2, CCL17, and CCL20 protein was quantified in chemokine-specific ELISA. CCL2 was secreted continuously and reached a concentration of 50 ng/mL in the supernatant (Figure 2B). CCL17 protein was under the detection limit of 50 pg/mL (data not shown). The concentration of endothelial CCL20 reached a maximum of 1 ng/mL after 6 hours of activation and did not accumulate further (Figure 2B). The protein concentration in the supernatant followed the mRNA level with a delay of 4 hours (Figure 2B). These expression data confirm and extend studies of human endothelial cells.16,18 Our results indicate for the first time in a murine model a continuous and inducible secretion of CCL2. CCL17 is not detectable in the supernatant of endothelial cells despite the presence of mRNA after activation. The protein has also not been detected in immunohistologic staining of activated bEnd.3 cells (data not shown). This could be due to a lack of secretion of this chemokine and points to a posttranscriptional regulatory mechanism as it was described recently for CCL5 produced by T cells.19 Finally, the secretion of CCL20 is strongly up-regulated upon induction with proinflammatory cytokines.
Analysis of chemokine and chemokine receptor expression and function. (A) Transcription of CCL2, CCL17, and CCL20 was quantified by real-time PCR. To monitor the amplification of the specific cDNA at the indicated points of time, the fluorescence of PCR products caused by intercalated SYBR-Green was determined. The specifically amplified cDNA was quantified in relation to β-actin transcripts from the same cDNA preparation and is shown as x-fold induction of the gene transcripts of activated cells compared with nonactivated cells. At the bottom, an agarose gel separation of the corresponding product is shown. (B) The concentration of CCL2 (left) and CCL20 (right) in the supernatant of stimulated cells was determined at the indicated points of time using a chemokine-specific ELISA. The detection limit of the CCL2 and the CCL20 ELISAs was 4 pg/mL and 150 pg/mL, respectively. (C) The expression of CC receptor transcripts on sorted marginal zone (CD21+ CD23–) and naive follicular B cells (CD21+ CD23+) was analyzed by RT-PCR. (D) The surface expression of CCR6 after ligand binding was analyzed using flow cytometry. Splenocytes were incubated with an anti-CCR6 mAb for 20 minutes at 4°C. Subsequently, the ligand CCL20 was added and the cells were incubated at room temperature. After the times indicated, the cells were placed on ice anda secondary antibody was used to reveal the CCR6 expression on naive (CD21+ CD23+) follicular B cells (bold line indicates CCR6 expression on naive untreated cells; thin line, CCR6 expression of B cells after binding of CCL20; and broken line, isotype). (E) The chemokines CXCL12 (2; 10 ng/mL) and CCL20 (3; 100 ng/mL) were used to induce locomotion in CCR6-positive (▪) and CCR6-negative (□) B cells in a transwell assay. Cells were allowed to migrate for 2 hours at 37°C, harvested from the lower chamber, and analyzed by flow cytometry. As controls, no CCL20 was added to the lower chamber (1, medium control). Furthermore, as desensitization controls B cells were preincubated (10 minutes) with CCL20 (100 ng/mL) before being added to the upper chamber with (4) or without (5) the chemokine. The results of 3 independent experiments are presented as mean ± SEM. Statistical significance was determined using an unpaired Student t test (*P ≤ .001, **P ≤ .001, ***P ≤ .002). (F) The adhesion of B cells to endothelial cells activated for 6 hours was determined after Ptx treatment or desensitization of splenocytes with recombinant CCL20 at a concentration of 10 ng/mL and 1000 ng/mL. The number of B cells was quantified using electronic gating. Adhesion of untreated B lymphocytes to activated endothelial cells was set at 100% (*P ≤ .001, **P ≤ .004, ***P ≤ .005). The results of 4 independent experiments are presented as mean ± SEM. Statistical significance was determined using an unpaired Student t test.
Analysis of chemokine and chemokine receptor expression and function. (A) Transcription of CCL2, CCL17, and CCL20 was quantified by real-time PCR. To monitor the amplification of the specific cDNA at the indicated points of time, the fluorescence of PCR products caused by intercalated SYBR-Green was determined. The specifically amplified cDNA was quantified in relation to β-actin transcripts from the same cDNA preparation and is shown as x-fold induction of the gene transcripts of activated cells compared with nonactivated cells. At the bottom, an agarose gel separation of the corresponding product is shown. (B) The concentration of CCL2 (left) and CCL20 (right) in the supernatant of stimulated cells was determined at the indicated points of time using a chemokine-specific ELISA. The detection limit of the CCL2 and the CCL20 ELISAs was 4 pg/mL and 150 pg/mL, respectively. (C) The expression of CC receptor transcripts on sorted marginal zone (CD21+ CD23–) and naive follicular B cells (CD21+ CD23+) was analyzed by RT-PCR. (D) The surface expression of CCR6 after ligand binding was analyzed using flow cytometry. Splenocytes were incubated with an anti-CCR6 mAb for 20 minutes at 4°C. Subsequently, the ligand CCL20 was added and the cells were incubated at room temperature. After the times indicated, the cells were placed on ice anda secondary antibody was used to reveal the CCR6 expression on naive (CD21+ CD23+) follicular B cells (bold line indicates CCR6 expression on naive untreated cells; thin line, CCR6 expression of B cells after binding of CCL20; and broken line, isotype). (E) The chemokines CXCL12 (2; 10 ng/mL) and CCL20 (3; 100 ng/mL) were used to induce locomotion in CCR6-positive (▪) and CCR6-negative (□) B cells in a transwell assay. Cells were allowed to migrate for 2 hours at 37°C, harvested from the lower chamber, and analyzed by flow cytometry. As controls, no CCL20 was added to the lower chamber (1, medium control). Furthermore, as desensitization controls B cells were preincubated (10 minutes) with CCL20 (100 ng/mL) before being added to the upper chamber with (4) or without (5) the chemokine. The results of 3 independent experiments are presented as mean ± SEM. Statistical significance was determined using an unpaired Student t test (*P ≤ .001, **P ≤ .001, ***P ≤ .002). (F) The adhesion of B cells to endothelial cells activated for 6 hours was determined after Ptx treatment or desensitization of splenocytes with recombinant CCL20 at a concentration of 10 ng/mL and 1000 ng/mL. The number of B cells was quantified using electronic gating. Adhesion of untreated B lymphocytes to activated endothelial cells was set at 100% (*P ≤ .001, **P ≤ .004, ***P ≤ .005). The results of 4 independent experiments are presented as mean ± SEM. Statistical significance was determined using an unpaired Student t test.
To investigate the potential of B cells to interact with endothelial chemokines, the expression of chemokine receptor mRNA was analyzed. Both MZ and follicular B cells displayed strong expression of the receptor CCR2, which is specific to CCL2, and the receptors CCR4 and CCR8, which are specific to CCL17. In contrast, the CCL20 receptor, CCR6, was only expressed at high level in follicular B cells (Figure 2C). This finding is in agreement with an earlier study that demonstrated a strong expression of CCR6 on the cell surface of follicular B cells and, consequently, a migratory response of naive B cells to CCL20.20 This implies a unique role for the inducible chemokine CCL20 in our system.
To test the specific activity of CCL20 and to exclude an interaction of the chemokine with receptors other than CCR6, a flow cytometric analysis of CCR6 after binding of the ligand and a transmigration assay were performed. A desensitization of CCR6 with CCL20 resulted in a down-regulation of the receptor from the cell surface (Figure 2D). The down-regulation was clearly visible after 10 minutes, reached a maximum after 30 minutes, and remained constant for at least 1 hour. In a transwell chamber wild-type B cells migrated in response to CCL20 whereas no migration was observed with CCR6-deficient B cells. A desensitization of CCR6 prevented the migratory response (Figure 2E). CCL2 and CCL17 did not induce migration of B cells (data not shown). Only 20% of B cells transmigrated in response to CCL20. Adding more CCL20 did not increase this proportion of migrating cells (data not shown). This could indicate a new distinct minor B-cell population that exhibits a functional CCR6. The experiment demonstrates the exclusive interaction of CCL20 with CCR6 and confirms earlier reports that CCR6 is the sole receptor of CCL20.21 Furthermore, it shows that murine B cells migrate in a CCL20 gradient20 even though CXCL12 is more potent. This is consistent with earlier studies that also showed that a small fraction of naive B cells responded to CCL20 and migrated in a gradient of this chemokine.22,23 There is, however, one differing study that demonstrates that CCL20 does not induce locomotion of human tonsil B cells.24 This discrepancy can be explained because in the study purified B cells from inflamed tonsils were used indiscriminately for the assays, whereas the studies mentioned above and our work were focused specifically on phenotypically naive B cells. The B cells harvested from inflamed tissue probably are activated and therefore are different from naive follicular B cells. This view is supported by the ability of these B cells to migrate in response to CCL2, an inflammatory chemokine.24
In addition, recombinant CCL20 was used to desensitize CCR6-mediated signaling in an adhesion assay. This treatment reduced the number of adherent B cells at concentrations of 10 ng/mL and 1000 ng/mL significantly by 25% (Figure 2F), indicating a saturating amount of CCL20 already at 10 ng/mL. The chemokines CCL2 and CCL17 were also tested in our assay, but desensitization of B cells with these chemokines did not interfere with adhesion (data not shown). The fact that Ptx blocked consistently 60% of the B-cell adhesion in this assay argues for the contribution of at least one more G protein–dependent mechanism. A subpopulation of human T cells, CCR6-positive memory T cells, has been shown to bind to activated endothelial cells.16 In this study the Ptx-dependent block of adhesion is mirrored in full by CCL20 desensitization. It is therefore also conceivable that a CCL20-mediated interaction of B cells with the endothelium functionally defines a distinct minority of B cells.
In conclusion, we demonstrate that naive B cells preferentially adhere to endothelial cells in an inflammatory situation and this adhesion is under partial control of the endothelial CCL20 and its receptor, CCR6. Furthermore, we show in a transmigration experiment that CCR6 is the only receptor of CCL20. CCR6-deficient B cells do not migrate in response to CCL20. The physiologic role of the striking adhesion of CCR6+ naive B cells to activated endothelial cells is not yet defined. However, there is evidence in chronic inflammatory models that expression of CCL20 can be correlated with the infiltration of B cells. Synovial fibroblasts express large amounts of CCL20 in rheumatoid arthritis25 and support the infiltration of CCR6+ leukocytes. A major part of the rheumatoid infiltrate consists of B cells, and the pathophysiologic role of these cells has been discussed.26,27 It is tempting to speculate that inflammatory CCL20 attracts B cells into the synovium and acts together with other chemokines and cytokines in the perpetuation of the inflammatory process.28
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2003-01-0007.
Supported by grants from the Deutsche Forschungsgemeinschaft (DFG-750, OZ; Ko-1315/7-1 [H.K.]) and by the BMBF (IZKF NW 1 [H.K.]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Karen Vana and Dr Peter Rohwer for their technical help and Dr Matthias Clauss for critical reading of the manuscript.