Abstract
Platelets regulate new blood vessel growth, because they contain a number of angiogenesis promoters and inhibitors. Additionally, platelets contain matrix metalloproteinases (MMPs), which when released mediate platelet adhesion and aggregation, and plasminogen, a fibrinolytic system enzyme that serves to limit blood clot formation. Enzymatic cleavage of plasminogen by MMPs generates angiostatin, an angiogenesis inhibitor. Therefore, we examined whether platelets generate angiostatin during aggregation in vitro. Platelets were isolated from healthy human donors and then aggregated with collagen, thrombin, or HT-1080 fibrosarcoma cells. Angiostatin was detected by Western blot analysis in the platelet releasates of all blood donors irrespective of the aggregating agent used. Platelet pellet homogenates showed the presence of angiostatin in all donors, which was released upon aggregation. Furthermore, platelet-derived angiostatin was isolated and purified by lysine-Sepharose affinity chromatography from collagen-aggregated platelet releasates. Bioassay of platelet-derived angiostatin showed that it inhibited the formation of capillary structures by human umbilical vein endothelial cells (HUV-EC-Cs) in an in vitro angiogenesis model. Inhibition of angiostatin in platelet releasates promoted the formation of capillary structures by HUV-EC-Cs. We conclude that healthy human platelets contain angiostatin, which is released in active form during platelet aggregation, and platelet-derived angiostatin has the capacity to inhibit angiogenesis.
Introduction
Platelets play a vital role in maintaining hemostasis by forming white aggregates that seal breaches in vascular integrity. In addition to preventing bleeding, platelets are known to play roles in nonhemostatic processes such as immunity1 and regulation of angiogenesis.2 Platelets contain a number of proangiogenic and antiangiogenic factors, which have the potential to regulate angiogenesis. Among the angiogenesis promoters found in platelets are vascular endothelial growth factor (VEGF)–A3 and –C,4 platelet-derived growth factor (PDGF),5 basic fibroblastic growth factor (bFGF),6 and epidermal growth factor (EGF).7 Furthermore, platelets contain matrix metalloproteinases (MMPs),8-10 which are also known to promote angiogenesis,11 and often are used as markers of the angiogenic process. To balance off the effects of the angiogenesis promoters, a number of angiogenesis inhibitors are also found in platelets. Included among this group are thrombospondin (TSP-1),12 platelet factor 4 (PF4),13 transforming growth factor-β1 (TGF-β1),14 and tissue inhibitors of matrix metalloproteinases (TIMPs).15 Interestingly TIMPs, endogenous inhibitors of MMPs, may stimulate or inhibit angiogenesis in a concentration- and tissue-specific manner.11 In addition to remodeling of the extracellular matrix, MMPs play a role in nonremodeling processes such as platelet aggregation.16 Other nonremodeling processes mediated by MMPs include the generation of the potent angiogenesis inhibitor angiostatin, discovered by O'Reilly and colleagues in 1994.17 Generation of angiostatin results from the cleavage of the plasminogen/plasmin molecule(s) by a number of MMPs, including MMP-2,18 MMP-3,19,20 MMP-7,20,21 MMP-9,20,21 and MMP-12.22 In addition to MMPs, a number of other proteases have been shown to generate angiostatin, including urokinase (uPA) and tissue-type plasminogen activator (tPA).23
The generation of angiostatin has been found primarily in tumor cells,24-27 although macrophages have also been shown to form angiostatin as a byproduct of the proteolytic regulation of membrane-bound plasmin.28 Interestingly, platelets have the ability to convert plasminogen to plasmin on their membrane surface.29,30 This conversion is mediated via tPA,31 one of the enzymes shown to generate angiostatin from plasminogen.23 Moreover, platelets not only express MMP-1, MMP-2, and MMP-9 activity, but also these MMPs are translocated to the membrane surface and released upon activation.8-10,32 Thus, platelets possess all the components required to generate angiostatin. Moreover, it has been observed that plasminogen-derived kringles may be found in normal human plasma33 ; however, the precise source of kringle generation has not been elucidated. All these data have prompted us to investigate the hypothesis that angiostatin is generated and released by normal human platelets upon platelet aggregation.
Materials and methods
Blood platelets
Approval for the current study was obtained from the University of Alberta institutional review board. Informed consent was provided according to the Declaration of Helsinki. Blood was collected from healthy volunteers who had not taken any drugs for 14 days prior to the study. Washed platelet suspensions (250 × 109/L [2.5 × 1011/L]) were prepared in Tyrode buffer as previously described.34
Platelet aggregation
Washed platelets were preincubated for 2 minutes at 37°C in a whole-blood ionized calcium lumi-aggregometer (Chronolog, Havertown, PA). Platelet aggregation was then initiated by the addition of collagen (5 μg/mL), thrombin (0.1 U/mL), or HT-1080 cells (1 × 104/mL) and monitored by Aggro-Link software for 15 minutes as previously described.15,35,36 After aggregation, platelet pellets were separated from releasates using centrifugation (1800g for 10 minutes) and then stored at –80°C until assayed for the presence of angiostatin by Western blot analysis.
Western blotting
Western blot analysis was performed as described previously.15 Briefly, to study angiostatin content in stimulated and unstimulated platelets, the platelet pellets were sonicated, centrifuged at 10 000g for 20 minutes at 4°C, and the resultant supernatants (150 μg protein per lane) were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). To study the release of angiostatin by aggregating platelets, platelet releasates were concentrated at 4°C by spin concentrators with a molecular weight cutoff of 10 000 Da; the concentrated releasates were then subjected to 12% SDS-PAGE (40 μg protein per lane). Following electrophoresis and transfer of samples onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA), the blots were blocked overnight in blocking buffer and then incubated with the primary antibody (0.8 μg/mL) for 2 hours. An antigoat horseradish peroxidase–labeled antibody was used as the secondary antibody (0.8 μg/mL) (Sigma, Oakville, ON, Canada). The immunoreactive bands were visualized with an ECL Plus kit (Amersham Biosciences, San Francisco, CA).
Cell culture
HT-1080 fibrosarcoma and HUV-EC-C human umbilical cord endothelial cell lines (-C ATCC designation) were obtained from the American Type Culture Collection (Rockville, MD). The HT-1080 cell line was cultured as monolayers in 25-cm2 culture flasks at 37°C in a humidified atmosphere with 5% CO2. The HT-1080 cells were cultured in 90% Dulbecco modified eagle medium (DMEM) with gentamicin (0.05 mg/mL), penicillin (0.06 mg/mL), streptomycin (0.01 mg/mL), and 10% fetal bovine serum (FBS). The cells were supplied with fresh medium and subcultured 3 times each week. Cells were detached from the flasks using EDTA (ethylenediaminetetraacetic acid) (7 mM) in DMEM with 10% FBS and gentle shaking as previously described.15,35,36 EDTA was then washed away with Tyrode solution, and the cells were resuspended in Tyrode solution at a concentration of 106/mL.
The HUV-EC-C cell line was cultured as monolayers in 75-cm2 culture flasks at 37°C in a humidified atmosphere with 5% CO2. The cells were cultured in 90% Kaighn F12K medium with gentamicin (0.05 mg/mL), penicillin (0.06 mg/mL), streptomycin (0.01 mg/mL), and 10% FBS. Furthermore, the medium was supplemented with 0.1 mg/mL heparin and 0.03 mg/mL endothelial cell growth supplement (ECGS) (Sigma). The cells were supplied with fresh medium every 2 days and subcultured once a week. The cells were detached from flasks using a trypsin-EDTA solution. Following harvesting, HUV-EC-C cells were resuspended in Kaighn F12K medium and counted. Cell concentration was then adjusted to 106 cells per milliliter.
Isolation of angiostatin: affinity chromatography
Platelets were isolated from 4 individuals and were resuspended as previously described at (250 × 109/L [2.5 × 1011/L]). Subsequently, they were aggregated with 5 μg/mL collagen, and the resulting releasates were concentrated at 4°C by spin concentrators with a molecular weight cutoff of 10 000 Da to decrease the volume required to load onto the column for affinity chromatography. Following concentration the samples were pooled and applied to a lysine-Sepharose column (Amersham Biosciences),37,38 which was packed and equilibrated with 3 bed volumes of 50 mM phosphate binding buffer, pH 7.5. Weakly bound proteins were eluted with 3 bed volumes of 50 mM phosphate binding buffer and 0.5 M NaCl. Samples were eluted with 0.2 M ϵ-aminocaproic acid and collected as 1 mL fractions. All fractions were assayed for protein, and those containing protein were pooled and concentrated using spin concentrators at 4°C as described in this paragraph. Samples of the concentrated fractions were then subjected to analysis via 12% SDS-PAGE followed by detection of protein bands by Coomassie brilliant blue staining.
Concentrated fractions were further concentrated using vacuum centrifugation and then diluted with phosphate-buffered saline (PBS). Final concentrations of fractions were determined by densitometric analysis of 12% SDS-PAGE Coomassie brilliant blue–stained gels containing angiostatin standards and experimental fractions. Angiostatin concentrations in experimental fractions were read using linear regression analysis (Graph Pad Prism 3.0).
Angiogenesis assay
A commercially available in vitro angiogenesis assay kit was purchased from Chemicon International (Temecula, CA). The assay was carried out as per assay instructions with the following modifications. HUV-EC-C cells were seeded in duplicate (5 × 104 cells per well) onto the surface of the polymerized ECMatrix in the absence of FBS in the medium. Platelet-derived angiostatin or angiostatin standard (0.03 to 3 μg/mL) was then added to HUV-EC-C cells and ECMatrix. HUV-EC-C cells and ECMatrix as control, or HUV-EC-C cells and ECMatrix with vehicle control (0.05% ϵ-aminocaproic acid in PBS), or with platelet-derived angiostatin or angiostatin standard (0.3-3 μg/mL) were all incubated for 8 hours at 37°C in a humidified atmosphere with 5% CO2. Cellular network structures began to develop after 4 hours. After 8 hours when the network structures were fully developed the networks were examined microscopically, fixed, and stained with a 0.5% crystal violet stain. Angiogenesis was quantitated by counting network branch points. The numbers of branch points in duplicate wells were averaged, and then this average was reported for 3 independent experiments.
To determine the effects of platelet releasates on angiogenesis and the effects of angiostatin in platelet releasates, angiogenesis assays were performed as described in the previous paragraph except for the following changes. Following detachment from their flasks, HUV-EC-C cells underwent 3 washes with Tyrode buffer to remove any growth factors found in medium, and finally HUV-EC-C cells were resuspended in Tyrode buffer (106 cells per milliliter). HUV-EC-C cells were added to platelet releasates stimulated by 0.1 U/mL thrombin. To study the effect of angiostatin in platelet releasates, these releasates were preincubated overnight at 4°C with 4 μg/mL antiangiostatin antibody (R&D Systems, Minneapolis, MN) or 4 μg/mL goat immunoglobulin G (IgG) (Sigma) as isotype control. HUV-EC-C cells suspended in platelet releasates were seeded in duplicate at 5 × 103 cells per well onto the surface of the polymerized ECMatrix. All these steps were performed to minimize the strong proangiogenic effect of the ECMatrix.
Microscopy
Microscopy was performed using a Zeiss Axiovert S100 microscope equipped with a Cool Snap-Pro camera (Media Cybernetics, Silver Spring, MD) and captured using Image Pro Plus software. Additional microscopy was performed using an Olympus CKX41 microscope equipped with a digital camera, and the images were captured using MicroFire software. All images were adjusted for contrast and brightness using Adobe Photoshop 5.0 software but otherwise not manipulated in any manner.
Reagents
If not otherwise specified, all reagents in the study were purchased from Sigma. Collagen and thrombin were obtained from Chronolog. Two antihuman angiostatin antibodies were used. The first was an antirecombinant human angiostatin affinity-purified polyclonal antibody (AF226) (R&D Systems), and the second was an antirecombinant human angiostatin monoclonal antibody (Clone Angio30 1-126) (Oncogene, San Diego, CA). While both antibodies recognized angiostatin in our experiments, the R&D Systems antibody was used in all subsequent experiments because it gave a stronger immune signal. Human angiostatin protein was obtained from Oncogene.
Statistics
Statistics were performed using Graph Pad Software Prism 3.0. All means are reported with standard error. One-way analysis of variance (ANOVA) with Tukey-Kramer multiple comparisons test and, also, paired and unpaired t tests were performed where appropriate. A P value of less than .05 was considered significant.
Results
Platelet aggregation
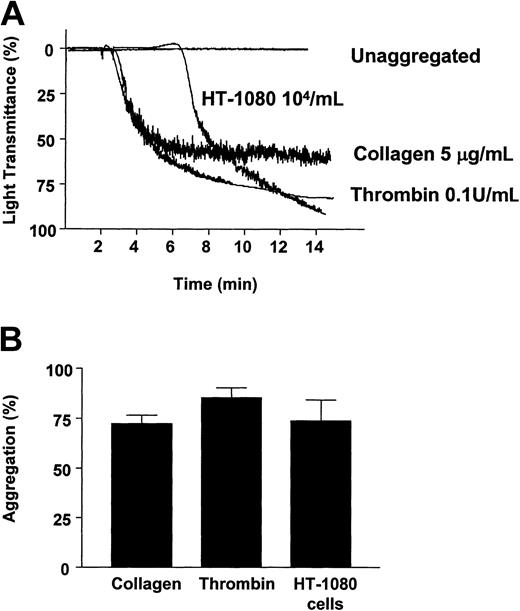
Platelets were aggregated with collagen (5 μg/mL), thrombin (0.1 U/mL), and HT-1080 fibrosarcoma cells (104/mL) as previously described15,34,35 (Figure 1A) and resulted in platelet aggregations of 72.25% ± 4.50% (n = 4), 85.25% ± 5.20% (n = 4), and 73.75% ± 10.57% (n = 4), respectively (Figure 1B).
Induction of platelet aggregation by collagen (5 μg/mL), thrombin (0.1 U/mL), and HT-1080 cells (104/mL). Representative traces (A) and summarized data (B). Bars are means ± SE from 4 experiments.
Induction of platelet aggregation by collagen (5 μg/mL), thrombin (0.1 U/mL), and HT-1080 cells (104/mL). Representative traces (A) and summarized data (B). Bars are means ± SE from 4 experiments.
Detection of angiostatin in platelets and their releasates
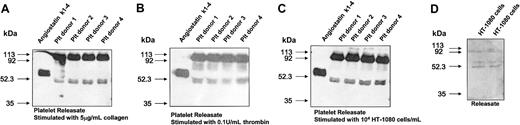
Platelet releasates from platelets aggregated with collagen, thrombin, and HT-1080 cells were collected and subjected to immunoblotting for the presence of platelet-derived angiostatin (Figure 2A-C). Platelet releasates from all donors showed angiostatin immunoreactive bands at approximately 52 kDa molecular weights for all 3 aggregation experiments. These bands migrated slightly below the angiostatin K1-4 standards. HT-1080 cells also showed immunoreactive bands for the presence of angiostatin in cultured medium (Figure 2D), indicating that HT-1080 cells generate this protein.
Western blot analysis. Analysis for platelet-derived angiostatin from 4 donors in releasates from platelets (Plt) aggregated with 5 μg/mL collagen (A), 0.1 U/mL thrombin (B), and 104/mL HT-1080 cells (C). Western blot analysis for angiostatin in the medium of cultured HT-1080 cells (D).
Western blot analysis. Analysis for platelet-derived angiostatin from 4 donors in releasates from platelets (Plt) aggregated with 5 μg/mL collagen (A), 0.1 U/mL thrombin (B), and 104/mL HT-1080 cells (C). Western blot analysis for angiostatin in the medium of cultured HT-1080 cells (D).
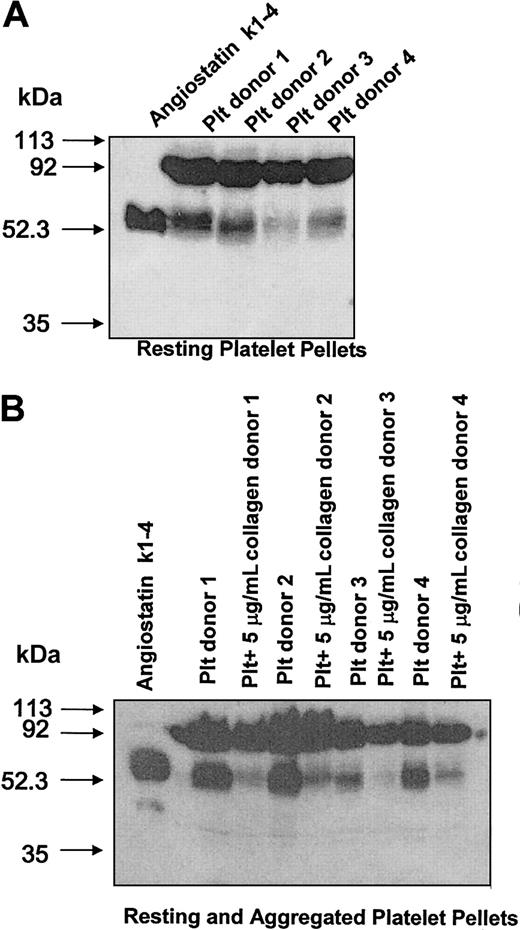
To determine if resting platelets contained angiostatin, unaggregated platelet pellets were collected and immunoblotted for the presence of angiostatin. Platelet pellets from 4 donors showed a distinct angiostatin immunoreactive band at approximately 52 kDa that comigrated with angiostatin standard (Figure 3A). Upon aggregation, platelets released intraplatelet angiostatin as evidenced by the decrease of angiostatin in aggregated, as compared with resting, platelet pellets (Figure 3B). Moreover, the release of angiostatin was associated with a decrease in plasminogen levels at 92 kDa in aggregated platelet pellets as compared with resting platelet pellets (6291 ± 1159 vs 7713 ± 872 arbitrary units of density per milligram of protein, respectively; P = .0101).
Western blot analysis for platelet angiostatin from platelet pellets of 4 donors.
Western blot analysis for platelet angiostatin from platelet pellets of 4 donors.
Isolation of platelet-derived angiostatin from platelet releasates
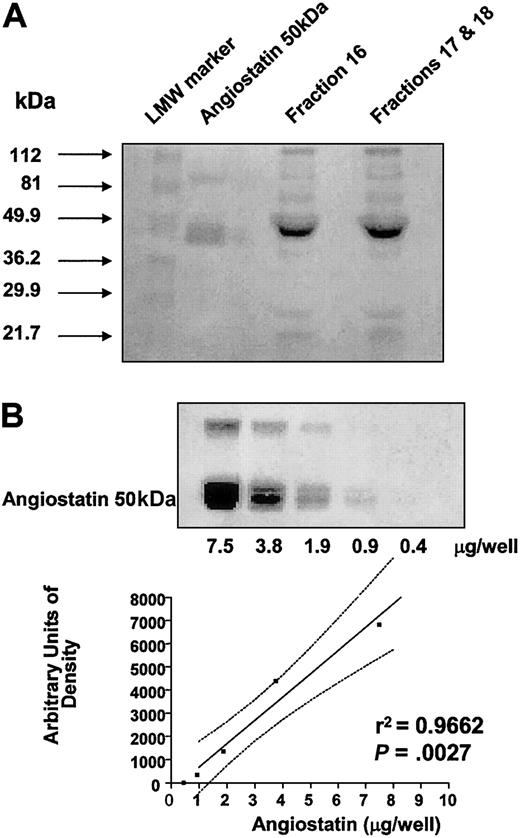
A total of 200 mL blood was obtained from 4 healthy human volunteers. From these 4 individuals, platelets were isolated and resuspended at 250 × 109/L (2.5 × 1011/L) and then subjected to aggregation with 5 μg/mL collagen in the same fashion as described in the legend to Figure 1A. Platelet releasates were then concentrated and pooled for angiostatin isolation using lysine-Sepharose affinity chromatography. Isolated fractions were concentrated and tested for the presence of angiostatin by SDS-PAGE followed by staining of the gel with Coomassie brilliant blue. Affinity-chromatographed fractions migrated with 50 kDa angiostatin standard to an approximate molecular weight of 49.9 kDa (Figure 4A).
Platelet-derived angiostatin. (A) Coomassie brilliant blue–stained SDS-PAGE of fractions 16 and pooled fractions 17 and 18. Fractions were obtained by lysine-Sepharose affinity chromatography for angiostatin of pooled platelet releasates. (B) Linear regression analysis (plotted points and solid line; confidence interval, dotted lines) of 50 kDa angiostatin standard used to determine concentrations of affinity-purified platelet-derived angiostatin fractions.
Platelet-derived angiostatin. (A) Coomassie brilliant blue–stained SDS-PAGE of fractions 16 and pooled fractions 17 and 18. Fractions were obtained by lysine-Sepharose affinity chromatography for angiostatin of pooled platelet releasates. (B) Linear regression analysis (plotted points and solid line; confidence interval, dotted lines) of 50 kDa angiostatin standard used to determine concentrations of affinity-purified platelet-derived angiostatin fractions.
Concentrations of platelet-derived angiostatin, in concentrated affinity-chromatographed fractions, were in the range of 1 to 5 mg/mL, as calculated from regression analysis of angiostatin standards (Figure 4B).
Inhibition of endothelial cellular network formation by platelet-derived angiostatin
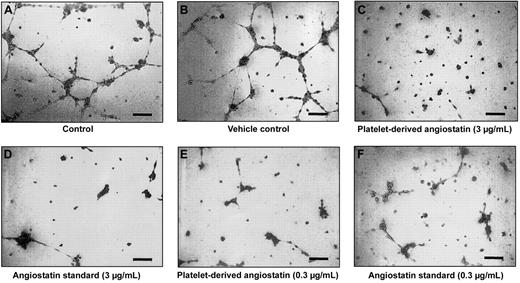
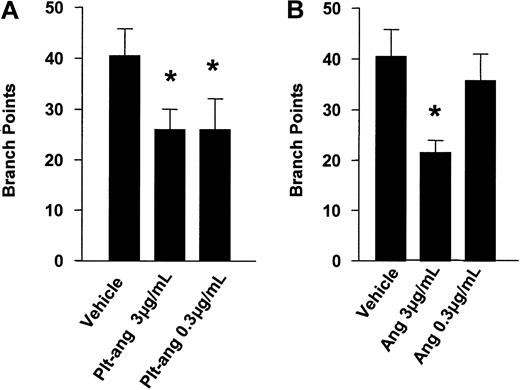
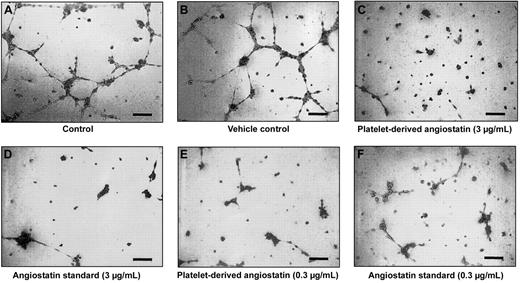
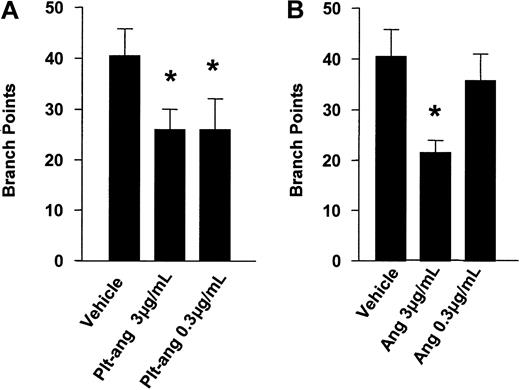
Platelet-derived angiostatin was tested for its ability to inhibit HUV-EC-C cells from forming capillary tubes and cellular network structures. After 8 hours of incubation on ECMatrix, HUV-EC-C cells alone and HUV-EC-C cells treated with vehicle formed extensive network structures of capillary tubes (Figure 5A-B). Both HUV-EC-C cells treated with platelet-derived angiostatin (0.3 and 3 μg/mL) and 50 kDa angiostatin standard (0.3 and 3 μg/mL) inhibited the formation of capillary tube network structures (Figure 5C-F). The reduction in capillary tube network formation by platelet-derived angiostatin and 50 kDa angiostatin standard was quantitated by counting the number of sprouted branch points in each well of 96-well plates. Both 50 kDa angiostatin standard and platelet-derived angiostatin inhibited angiogenesis (Figure 6). The extent of this inhibition was similar with the 2 preparations of angiostatin.
An endothelial capillary tube formation assay was used to test the effects of platelet-derived angiostatin. A total of 5 × 104 HUV-EC-C cells were plated out per well, resulting in tube formation. Microscopy of representative experiments: (A) control and (B) vehicle control. Effects of platelet-derived angiostatin and 50 kDa angiostatin standard on capillary tube formation: (C) 3 μg/mL platelet-derived angiostatin, (D) 3 μg/mL 50 kDa angiostatin standard, (E) 0.3 μg/mL platelet-derived angiostatin, and (F) 0.3 μg/mL 50 kDa angiostatin standard. Bar represents 100 μm.
An endothelial capillary tube formation assay was used to test the effects of platelet-derived angiostatin. A total of 5 × 104 HUV-EC-C cells were plated out per well, resulting in tube formation. Microscopy of representative experiments: (A) control and (B) vehicle control. Effects of platelet-derived angiostatin and 50 kDa angiostatin standard on capillary tube formation: (C) 3 μg/mL platelet-derived angiostatin, (D) 3 μg/mL 50 kDa angiostatin standard, (E) 0.3 μg/mL platelet-derived angiostatin, and (F) 0.3 μg/mL 50 kDa angiostatin standard. Bar represents 100 μm.
Inhibition of angiogenesis. Inhibition by platelet-derived angiostatin (A) and angiostatin standard (B). This was quantitated by counting branch points in each well of the endothelial capillary tube formation assay. Bars are means ± SE from 3 experiments. *P < .05 vehicle control versus treatments.
Inhibition of angiogenesis. Inhibition by platelet-derived angiostatin (A) and angiostatin standard (B). This was quantitated by counting branch points in each well of the endothelial capillary tube formation assay. Bars are means ± SE from 3 experiments. *P < .05 vehicle control versus treatments.
Effect of angiostatin inhibition in platelet releasates on angiogenesis
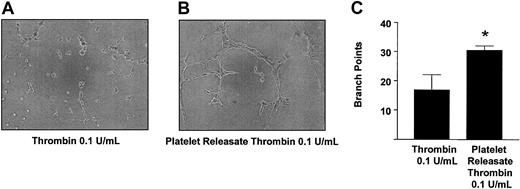
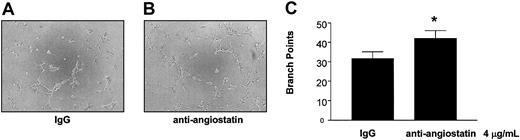
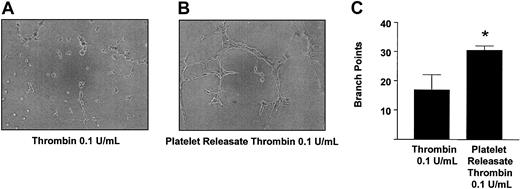
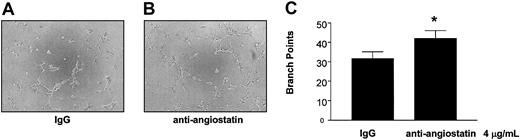
To examine further the physiological relevance of our findings, we studied the role of platelet-derived angiostatin on angiogenesis in platelet releasates. We chose thrombin to aggregate platelets, because it is the most potent platelet agonist known. The releasates of platelets aggregated with 0.1 U/mL thrombin stimulated the growth of capillary tube network structures to a greater extent as compared with control (P = .0177, n = 6) (Figure 7). Because the releasate of thrombin-aggregated platelets promoted the growth of capillary tube network structures, we next studied the effects of angiostatin inhibition in these releasates on angiogenesis. Preincubation of thrombin-aggregated platelet releasates with antiangiostatin antibody (4 μg/mL) resulted in an increase in the formation of capillary tube network structures as compared with thrombin-aggregated platelet releasates preincubated with IgG control (4 μg/mL) (P = .0285, n = 6) (Figure 8).
Promotion of angiogenesis by thrombin-aggregated platelet releasates. Microscopy of representative experiments (A-B) and statistical analysis (C). Bars are means ± SE from 4 and 6 experiments, respectively. *P < .05 vehicle control versus treatment. Original magnification of A and B, × 100.
Promotion of angiogenesis by thrombin-aggregated platelet releasates. Microscopy of representative experiments (A-B) and statistical analysis (C). Bars are means ± SE from 4 and 6 experiments, respectively. *P < .05 vehicle control versus treatment. Original magnification of A and B, × 100.
Potentiation of angiogenesis by inhibition of platelet-derived angiostatin. The release of angiostatin was stimulated with 0.1 U/mL thrombin. (A-B) Microscopy of representative experiments. (C) The statistical analysis. Bars are means ± SE from 6 experiments. *P < .05 control versus treatment. Original magnification of A and B, × 100.
Potentiation of angiogenesis by inhibition of platelet-derived angiostatin. The release of angiostatin was stimulated with 0.1 U/mL thrombin. (A-B) Microscopy of representative experiments. (C) The statistical analysis. Bars are means ± SE from 6 experiments. *P < .05 control versus treatment. Original magnification of A and B, × 100.
Discussion
Increasing evidence indicates that the hemostatic system, and more specifically platelets, plays a role in tumor angiogenesis.2,39-41 While most evidence has been accumulating for the proangiogenic effect of platelets with respect to tumor angiogenesis, little is known of the antiangiogenic properties of platelets that could potentially limit or counteract their ability to stimulate angiogenesis. Therefore, the objective of our investigation was to study whether platelets have the capacity to produce the potent angiogenesis inhibitor angiostatin, because they contain both the substrate (plasminogen) and the catalyst (MMPs) for its production.
First, using Western blot analysis we found the presence of angiostatin in the releasates of platelets from healthy human volunteers, indicating that angiostatin is released upon platelet aggregation. We used 2 platelet agonists, collagen and thrombin, to show that angiostatin generation by platelets is related to platelet aggregation. Furthermore, we used HT-1080 fibrosarcoma cells to study generation of angiostatin by platelets activated by tumor cells. It has been previously shown that tumor cell–induced platelet aggregation (TCIPA) is a powerful platelet stimulus that under pathological conditions may facilitate the embolization of the vasculature with tumor cells and in the formation of metastatic foci.35,36,42-44 The results of our current experiments show that angiostatin is liberated during TCIPA and that platelets are major contributors to the pool of this angiogenesis inhibitor. Interestingly, the relative amounts of angiostatin produced during TCIPA are similar to those produced during aggregation by collagen and thrombin. Thus, we postulate that platelet activation caused by soluble and cellular aggregating factors is associated with the release of angiostatin. In addition to platelet releasates, Western blot analysis was also performed on unaggregated platelet pellets. The immunoblot revealed the presence of angiostatin, indicating that platelets store angiostatin prior to its release. Moreover, the release of angiostatin from platelets was associated with a decrease of plasminogen levels in aggregated platelets, implicating platelet plasminogen as a potential substrate for angiostatin generation during platelet aggregation.
Because lysine binds plasminogen and its kringles37 and lysine-Sepharose affinity chromatography has been used to isolate and purify angiostatin,18,28,38,45 we have used lysine-Sepharose affinity chromatography to isolate and purify the inhibitor from platelet releasates. We have found that the protein, thus purified, has a similar molecular weight to the 50 kDa angiostatin standard, confirming the data obtained from Western blot experiments.
The major biologic characteristic of angiostatin is its ability to inhibit angiogenesis. We used this property of angiostatin to bioassay its effects on endothelial cellular network formation. As expected, purified platelet-derived angiostatin inhibited the formation of endothelial cellular networks. However, recently it has been observed that tissue factor (TF) can antagonize the inhibitory effects of angiostatin on endothelial cell proliferation46 and, importantly, that platelets contain TF in considerable amounts.47 Thus, the presence of angiostatin in the platelet releasate milieu alone does not imply that it will be in active form under physiological conditions. Therefore, we tested the effects of angiostatin blockade in platelet releasates on angiogenesis. We found that blockade of angiostatin in platelet releasates, using an antibody, resulted in an increase in the formation of capillary tube network structures. The apparent lack of an effect of platelet TF in blocking angiostatin activity in platelet releasates perhaps may be explained by the fact that activated platelets also release tissue-factor pathway inhibitor (TFPI).48 All our data clearly show that angiostatin is expressed by unaggregated human platelets and the inhibitor is released, in active form, during platelet activation.
We have also detected the presence of angiostatin in nonaggregated platelets. Because platelets acquire most of their proteins during fragmentation of large mother cells, megakaryocytes,49 it is likely that angiostatin found in nonaggregated platelets is synthesized by megakaryocytes and then passed onto platelets.
Interestingly, platelet aggregation leads to release of lower molecular weight angiostatin, suggesting that proteolytic processing is involved in this process. As mentioned previously, at least 7 different proteases (MMP-2, MMP-3, MMP-7, MMP-9, MMP-12, uPA, and tPA) have been shown to have the capability of generating angiostatins from plasminogen/plasmin,18-23 of which platelets are known to contain MMP-2, MMP-3, MMP-9, and tPA. Elucidation of their mechanisms may lead to a way of regulating the generation of platelet-derived angiostatin.
The finding that platelets express angiostatin is of significance for human physiology and pathology. Indeed, angiostatin has been found in a number of human fluids and tissues from both diseased26,50-53 and healthy humans.54 We propose that the endogenous generation of angiostatin in tumor-bearing animals and humans may also be partially due to an activated hemostatic system perhaps due to TCIPA.
The physiological role of platelets in regulating angiogenesis is only now beginning to be elucidated. As mentioned previously, platelets have been shown to possess many proangiogenic factors, such as VEGF, PDGF, bFGF, EGF, and MMPs, and also many antiangiogenic factors, such as TSP-1, PF4, TGF-β1, TIMPs, and angiostatin. Upon platelet activation and aggregation, a balance between proangiogenic and antiangiogenic factors must exist both to stimulate and to limit the growth of new blood vessels. This balance between platelet-derived angiogenesis-regulating factors may act in a similar manner to regulate angiogenesis as the balance between platelet proaggregotory factors, such as thromboxane (TXA2),55,56 adenosine diphosphate (ADP),57 and MMP-2,8 and antiaggregatory factors, such as prostacyclin (PGI2),58 nitric oxide (NO),59,60 MMP-9,9 and TIMP-4,15 acts to maintain hemostasis. Evidence of this angiogenic balance found in platelets is supported by our finding that inhibition of angiostatin in thrombin-aggregated platelet releasates resulted in an increase in angiogenesis in vitro. Thus, even though thrombin-aggregated platelet releasates promoted the growth of capillary network structures, an underlying antiangiogenic component was present in the platelet releasates. This antiangiogenic component of the platelet releasates was mediated, at least in part, by angiostatin. Thus, under physiological conditions platelet-derived angiostatin may serve as a counterbalance to such potent proangiogenic molecules as VEGF, which are released from platelets upon activation.3,4,61,62 Furthermore, we hypothesize that the balance between platelet proaggregatory and antiaggregatory factors, along with cellular and extracellular matrix angiogenesis-regulating molecules at sites of vascular damage, determines the net angiogenic signal from platelet-derived angiogenesis-regulating factors. Thus, the net angiogenic outcome will then be determined by both the milieu of platelet and vascular signals.
Evidence for the pathological role of platelets in tumor angiogenesis is now also beginning to accumulate. Verheul and colleagues have shown that platelets store and transport VEGF61 and that patients with soft tissue sarcomas show the presence of platelet activation and elevated levels of VEGF.63 Moreover, the presence of increasing numbers of platelets stimulates the proliferation of endothelial cells64 and endothelial cell tube formation in vitro.65
Finally, the isolation of platelet angiostatin may have pharmacologic significance. Platelet activation and thrombosis associated with cardiovascular disease and cancer metastasis can be controlled by various antiplatelet drugs. A potential of these agents to regulate the release of platelet angiostatin needs to be carefully examined.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-02-0378.
Supported by a grant (MOP-14074) from the Canadian Institutes of Health Research (CIHR). P.J. was supported by a PhD studentship from CIHR-Canadian Thoracic Society and Lung Association-Alberta Lung Association. D.A. was supported by Secretaria de Estado de Educacion y Universidades, cofunded by the European Social Fund, and is a postdoctoral fellow of the Spanish Ministry of Education. S.C.-B. is a recipient of an FPI Fellowship of the Comunidad de Madrid, Spain. M.W.R. was supported by a CIHR Scientist Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Ada Chung for blood collections.