Epstein-Barr virus (EBV) is a tumorigenic herpes virus, which is associated with several human hematologic neoplasias such as Burkitt lymphoma, Hodgkin disease, and posttransplantation lymphoproliferative disease (PTLD). EBV-induced lymphoproliferative disease represents a broad spectrum, ranging from benign disorders to malignant non-Hodgkin lymphomas occurring mainly as complication of immunodeficiency. However, its acute development after conventional chemotherapy as treatment for another malignancy is a rare finding.
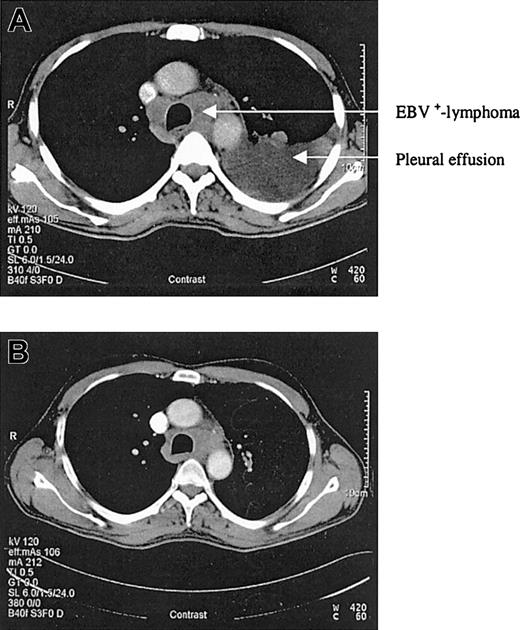
We report a case of acute EBV-associated B-cell diffuse large-cell lymphoma developing shortly after successful treatment of relapsed hairy cell leukemia. In 1998, a 46-year-old patient presented with splenomegaly, leukocytopenia, and thrombocytopenia. The peripheral blood smear demonstrated atypically appearing lymphocytes, resembling hairy cells. The bone marrow biopsy confirmed the diagnosis of hairy cell leukemia by May-Grunwald-Giemsa (MGG) staining, alkaline phosphatase antialkaline phosphatase (APAAP), and immunophenotyping with 85% cells positive for CD103. The patient was treated with continuous intravenous infusions of cladribine (3 mg/m2) for 7 days. His blood counts nearly normalized over the next months, and follow-up bone marrow biopsies confirmed a complete remission. However, in December 2002 a progressive leukocytopenia was detected and in January 2003 a relapse of the hairy cell leukemia was diagnosed by bone marrow biopsy with a 39% infiltration of the bone marrow. Cladribine was initiated again as salvage therapy for 7 consecutive days (3 mg/m2). At 3 weeks after completing the chemotherapy cycle, the patient developed fever, dyspnea, and cough. A computed tomography (CT) scan of the chest revealed typical aspergillosis infiltrates and pleural effusions. The aspergillus antigen in the serum, however, was always negative. Surprisingly, in several blood cultures and in the pleural effusion, high amounts of EBV DNA were detectable by polymerase chain reaction (115 000 genome equivalents/mL in the serum and 950 000 genome equivalents/mL in the pleural effusion). Broad spectrum antibiotics, voriconazole, and cidovir were initiated, leading to clinical improvement and regression of lung infiltrates and pleural effusions. To assess the pneumonia, a control CT scan was performed in April, which revealed regress of infection but also a new mediastinal mass and multiple new cervical lymph nodes (Figure 1A). The biopsy of a cervical lymph node revealed a CD20+ B-cell diffuse large-cell lymphoma that was EBV-associated with expression of latent membrane protein 1 (LMP-1) and EBV nuclear antigen 2 (EBNA-2+) (Figure 2). There was no sign of an infiltration by the hairy cell leukemia in the respective lymph node. At this time the hairy cell leukemia was in complete remission, measured by peripheral blood and bone marrow cytomorphology as well as multiparameter immunophenotyping. In this patient there was no previous history of congenital immunodeficiency. In addition, to exclude an HIV infection an HIV test was performed, which was negative. CD4 counts during this period of time, however, were not measured.As the infection was still not under definitive control and the patient's performance status was reduced, we felt that chemotherapy was not feasible. Therefore, a monotherapy with anti-CD20 monoclonal antibody (rituximab) was initiated at a dose of 375 mg/m2. Currently, the patient is in good general condition and follow-up CT scans revealed a good partial response 3 weeks after 4 applications of rituximab (Figure 1B).
CT scans before and after rituximab application. (A) CT scan of the chest in April 2003. (B) Control CT scan after 4 applications of rituximab showing a good partial response.
CT scans before and after rituximab application. (A) CT scan of the chest in April 2003. (B) Control CT scan after 4 applications of rituximab showing a good partial response.
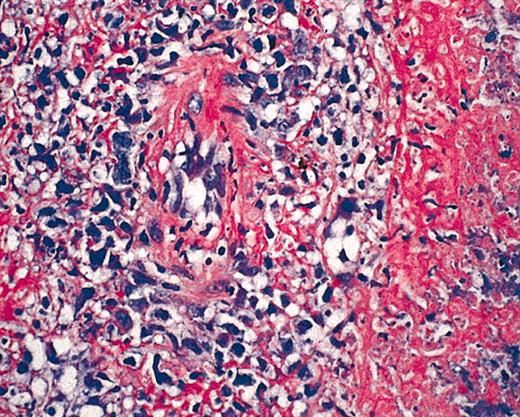
Hematoxylin and eosin (HE) staining of EBV+ B-cell diffuse large-cell lymphoma. Original magnification × 40.
Hematoxylin and eosin (HE) staining of EBV+ B-cell diffuse large-cell lymphoma. Original magnification × 40.
Patients with hairy cell leukemia are known to have an increased risk of developing secondary cancers,1 mainly solid tumors.2 These secondary neoplasias seem not to be associated with therapy of the disease or the following prolonged immunosuppression, but may also reflect the limited immune response capacity of patients with hairy cell leukemia per se. To our knowledge, an EBV-associated B-cell non-Hodgkin lymphoma developing in leukocytopenia as acute consequence of immunosuppression just shortly after treatment of hairy cell leukemia has not yet been described. The EBV status of our patient prior to the cladribine therapy is unknown, but the expression of both LMP-1 and EBNA-2 suggest a latency type III of EBV, a finding that is associated to immunodeficiency-associated lymphoproliferations. Currently, however, EBV DNA is no longer detectable.
The best management of patients with EBV-associated non-Hodgkin lymphoma is not fully elucidated. Conventional chemotherapy regimes or antiviral drugs are of limited efficacy. In contrast, a retrospective study and several case reports showed the effectiveness of rituximab monotherapy.3,4 However, these results have to be confirmed in prospective trials to further improve the prognosis of these patients.