Abstract
The gastrointestinal (GI) tract is a major target in graft-versus-host disease (GvHD). In rodents both tumor necrosis factor (TNF) and Fas-dependent apoptosis have been shown to play a major role in GvHD lesions, but data in humans on TNF and Fas in situ expression are scarce. More recently, the role of non-T cells as GvHD effectors has also been suggested in experimental models. Here we report a detailed quantitative pathologic analysis in 95 patients who underwent gastroduodenal biopsy. This analysis included characterization and quantification of the cellular infiltrate, TNF, TNF receptors, and Fas in situ expression analyses and quantification of apoptotic cell numbers. TNF was expressed in all biopsies and it was highly specific for acute GvHD. In multivariate analysis, including pathologic factors only, increased early transplantrelated mortality (TRM) was associated with the presence of more than 20 neutrophils per field. Factors affecting early and late TRM were then assessed by multivariate analyses including both pathologic and clinical factors. Increased day-90 TRM was associated with the presence of more than 5 apoptotic bodies per field within the cellular infiltrate, and with stage II or higher acute liver GvHD. One-year TRM associated with the same 2 factors and with chronic GvHD.
Introduction
Allogeneic stem cell transplantation (SCT) has been a successful clinical treatment modality for a variety of hematopoietic disorders and hematologic malignancies but graft-versus-host disease (GvHD) is still a major cause of posttransplantation mortality. Our understanding of the pathophysiology of GvHD has greatly improved with insights from experimental studies. Initial damage to host tissue is induced by the pretransplantation conditioning regimen.1-3 Donor T-cell activation, adhesion to and interaction with host tissue and costimulatory signals, and amplification of the cytokine network follow this initial damage. The effector phase leads to host-cell destruction via inflammatory molecules and cytolytic effects of activated T cells. The resultant damage in target organs includes apoptosis of epithelial cells with infiltrating immune cells in the vicinity of the apoptotic cell, a feature classically termed satellite cell “necrosis.” The gastrointestinal (GI) tract has a major role in developing GvHD.4 Release of inflammatory cytokines occurs primarily in the gut, which, when damaged by the conditioning regimen, allows the transfer of lipopolysaccharide (LPS) into the circulation and activation of macrophages. These events, in turn, further enhance the release of cytokines such as tumor necrosis factor α (TNF-α) and interleukin-1 (IL-1). Other studies5 showed that, in the effector phase6-8 Fas/Fas ligand and TNF/TNF receptors (TNFRs) both play a major role in GvHD.
Although T cells are important mediators of GvHD, the molecular mechanism they use to induce GvHD is controversial. Three effector pathways have been described for cytotoxic T lymphocytes: one requires perforin and granzymes, the second Fas (CD95) and its ligand, and the third TNF and its receptors to mediate cytotoxicity. Together, these mechanisms appear to account for virtually all cytotoxicity induced by activated cytotoxic T lymphocytes (CTLs) in standard in vitro assays. Finally, most recently, it was reported that T-cell expansion occurred early and contracted back to baseline by day 14, before the onset of GvHD mortality.9,10 Three weeks after transplantation, target organs are infiltrated by cells of the myeloid lineage, suggesting an important role for non-T cells as GvHD effectors.
In human beings, the relevance of such experimental studies is, however, far from clear. The early phases including antigen presentation, T-cell activation, and clonal expansion are most likely clinically nonapparent, and clinicopathologic diagnosis is made during the late phase of target-organ damage. In this prospective study involving 95 patients who underwent GI biopsy, we show that, in humans, both TNF-α and Fas are expressed in the infiltrate and that the number of apoptotic cells and granulocytes within the cellular infiltrate are highly predictive of transplant-related mortality (TRM).
Patients and methods
Patients
From June 1996 to December 1999, 95 patients with suspected GvHD of the GI tract (nausea/vomiting or diarrhea) were enrolled in this prospective study. Main patient, disease, and transplant characteristics are described in Table 1. The median age was 35 years (range, 5-60 years). Conditioning regimens varied with disease and disease stage, as previously described.11,12 Patients with myeloid malignancies received the association of total body irradiation (TBI) and cyclophosphamide with or without VP16, or the association of busulfan and cyclophosphamide with or without VP16. Patients with acute lymphoblastic leukemia (ALL) received TBI associated with melphalan and cytarabine (TAMe). Patients with aplastic anemia mostly received cyclophosphamide and antithymocyte globulins (ATGs). For the purpose of this study an intensified conditioning regimen was defined as any conditioning in which VP16 was added, or the TAMe regimen. The association of cyclosporine and methotrexate was used in 76% of the patients; and 12% of the patients (all receiving transplants from an unrelated donor) received a CD34+-selected graft for T-cell depletion.
Biopsies were performed as a diagnostic procedure to explore GI tract symptoms. Patients were informed, however, that part of the material could be used for research purpose. The ethical review board of the Hospital Saint Louis approved the protocol. Informed consent was provided according to the Declaration of Helsinki. The modified clinical staging and grading system of acute GvHD13 was used to assess disease severity, and chronic GvHD was classified as limited or extensive.
Methods
A uniform protocol was followed for all patients. The esophagus, the stomach, and the duodenum were systematically explored. Per protocol design, and for uniform analysis purposes, biopsy specimens analyzed were only those obtained from the second part of the duodenum. Biopsy procedures were systematically performed during fiberoptic examination of the upper intestinal tract. Two of them were immediately snap frozen in liquid nitrogen, 2 others were fixed in formalin and further processed for paraffin embedding, and one was immediately fixed in 2% glutaraldehyde in cacodylate buffer and further processed for resin embedding. On 3-μm-thick paraffin sections, hematoxylin-eosin, Masson trichrome, and May-Grünwald-Giemsa stains were systematically performed. In addition, 2 biopsies were sent to the microbiologic department for systematic search of viral (cytomegalovirus [CMV], adenoviruses, and enteroviruses) or fungal infection. The search for Clostridium difficile was also systematically performed in the stool.
On 5-μm-thick frozen sections, an automated indirect immunoperoxidase method (Nexes, Ventana Tucson, AZ) was used with primary antibodies directed against CD45RA (Dako, Glostrup, Denmark), CD68 (Dako), CD95 (Pharmingen, Heidelberg, Germany), TNF (Genzyme, Cambridge, United Kingdom), and both types of TNFRs (IgG monoclonal antibodies [MoAbs], TNFR 55 and TNFR 75, kind gift from Manfred Brockaus, Basel, Switzerland), at dilutions of 1:100, 1:50, 1:50, 1:200, 1:100, and 1:100, respectively. The specificity of immunohistochemistry was tested by omitting the first antibody and by substituting it with an irrelevant antibody of the same isotype, but with a different specificity. We failed to find any reliable antibodies to study FasL in human biopsies either by immunohistochemistry or in situ hybridization (data not shown) and thus our study relied only on the study of Fas expression in biopsies of the human GI tract, to study the Fas/FasL pathway.
On 5-μm-thick paraffin sections, after treatment with proteinase K (20 μg/mL) for 15 minutes at room temperature, a TUNEL (terminal desoxytransferase-catalyzed DNA nick-end labeling) assay was performed, to detect in situ fragmented DNA. The TUNEL assay was performed according to a previously published procedure.14 Samples fixed in glutaraldehyde were embedded in Epon and ultrathin sections were stained by uranyl acetate and lead nitrate and analyzed on a Jeol 100 B electron microscope (Leica, Rueil Malmaison, France). Ultrastructural studies focused on the analysis of apoptotic cells, their pattern of distribution, and their possible phagocytosis by surrounding cells.
Histologic criteria of digestive GvHD were assessed on the biopsies of upper GI tract. Sale et al15 proposed 4 grades for histologic criteria of digestive GvHD. Epstein et al16 added criteria of early GvHD, demonstrating that crypt cell degeneration, even without crypt dilatation and crypt abscess, is characteristic of GvHD. Washington et al17 showed that “crypt cell degeneration” corresponded to epithelial cell apoptosis or single-cell necrosis with karyorrhectic debris, and that it was the most useful marker of acute GvHD in the GI tract. Therefore, the histologic grading was as follows: grade I: crypt cell degeneration or epithelial cell apoptosis, without crypt loss; grade II: loss of up to 3 contiguous crypts; grade III: loss of 4 or more crypts without sloughing; and grade IV: total sloughing. The pattern of mucosal changes was determined blindly by 2 different pathologists, without knowledge of the clinical data, on similar areas in the different biopsies. In case of discrepancy between pathologists, a third analysis was made, and a consensus result was then established. Pathologic severity scores and analytical count of cellular populations are summarized in Table 2.
Statistical analysis
The pathologists (A.J. and M.D.) classified the biopsies as having evidence of (n = 71) or no pathologic evidence of GvHD (n = 24).
Among patients whose biopsies were performed before day 100 due to clinical symptoms possibly related to clinical acute GvHD (n = 78), the ability of elementary features to predict the existence of clinical GvHD was assessed through sensitivity, that is, the proportion of biopsies presenting the elementary feature among patients in whom the diagnosis of acute GvHD was retained (n = 65) and specificity, that is, the proportion of biopsies without the elementary feature among biopsies of patients who did not experience acute GvHD (n = 13).
Among the same patients was also assessed the ability of any feature to predict the occurrence of grade II or higher (n = 60) or grade 0 to I (n = 18) acute GvHD, and the occurrence of stage II or higher (n = 21) or 0 to I (n = 57) clinical intestinal GvHD. Confidence intervals (CIs) of sensitivity and specificity were calculated according to the exact sampling distribution. The ability of an elementary feature to predict the occurrence of each event was tested through the χ2 or Fisher exact test, when necessary.
To study the prognostic value of each elementary pathologic feature on TRM, the proportional hazard model with time-dependent covariates was used to take into account the variation of the timing of the biopsy procedure among patients.18 TRM at 90 days after transplantation was studied on a sample of 68 patients whose biopsies were performed before this time point. Twenty-one patients who died after day + 90 were censored alive at this time point. TRM at day 365 was studied on a sample of 94 patients. Four patients who died later were censored alive at 1 year. Similarly, 3 patients who died from relapse and 2 who had a relapse were censored at their time of death or relapse, respectively. To take into account the number of features tested (n = 25, described in Table 3), P < .002 was considered as significant. Prognostic factors were successively entered in the model following a forward procedure based on the likelihood ratio test without a priori selection of elementary features proposed to the model. For TRM study at 1 year, the occurrence of chronic GvHD, as a time-dependent covariate, was also proposed to the model. In TRM studies, the addition of clinical parameters to the final model was tested (recipient age, donor/recipient sex combination, recipient CMV serologic status, high-risk disease, intensified conditioning regimen, and unrelated transplants). Therefore, 4 multivariate prognostic models were derived, 2 including only biopsy elementary features significantly related to TRM (at day 90 or at 1 year), and 2 including both biopsy features and clinical parameters including acute GvHD or acute and chronic GvHD for TRM at day 90 and at 1 year, respectively. The same P (< .002) was used for significance.
Results
Clinical manifestations of GvHD
Acute and chronic GvHD. Upper GI tract biopsy was performed in all 95 patients with digestive symptoms with none or limited (< stage I) skin involvement, at the time of the biopsy. Biopsy specimens were collected in 78 (82%) patients within 100 days of bone marrow transplantation (BMT) and 17 (18%) after day 100. One patient had a complication (hemorrhage of the duodenal wall) resulting from the endoscopic procedure. The subsequent hematoma lasted for 4 weeks.
Acute GvHD. Overall, 78 of the 95 patients (82%) developed acute GvHD. The maximum clinical grades of acute GvHD were grade I for 11 patients, grade II for 38 patients, grade III for 14 patients, and grade IV for 15 patients. Median time to acute GvHD was 24 ± 3 days. Overall, 67 patients had severe acute GvHD of grade II or higher. Only 32 patients had stage II or higher skin involvement at any time before day 100. Similarly, the prevalence of stage II or higher liver GvHD was low (n = 22).
In patients who underwent biopsy before day 100 (n = 78), 65 developed acute GvHD (83%) and most of them (60 of 65, 92%) had grade II or higher. Only 21 patients had stage II or higher GI disease. Subsequently, 27 of these 65 patients also had stage II or higher skin disease and 22 had stage II or higher liver disease. Only 5 had persistent nausea and vomiting and positive pathologic findings as sole evidence of acute GvHD (ie, grade I acute GvHD of the upper GI tract, per international criteria). Among these 65 patients, 39 (60%) underwent biopsy within the first 4 to 5 days of GI symptoms as the first, initial sign of acute GvHD. Seven additional patients who had first limited (stage I-II) skin disease, underwent upper GI biopsies 0 to 4 days following the onset of GI symptoms (median, 2.5 days), and 7 to 31 days (median, 17.5 days) following the onset of the first sign of cutaneous GvHD. In 5 of these 7 patients, the digestive symptoms appeared while they were tapering steroids. Six patients had biopsy for GI symptoms 9 to 102 days (median, 60 days) but had no evidence of any pathologic sign of GvHD. Finally, 13 patients had late biopsy because of worsening of GI symptoms (n = 8; elapsed time from GvHD onset 12 days [range, 9-23 days]) or late flare of acute digestive GvHD, without initial biopsy (n = 5; elapsed time from GvHD onset 65 days [range, 37-98 days]).
Acute GvHD treatment consisted of steroids in all patients (starting dose, 2 mg/kg/d); 8 patients (6%) required additional drugs. At the time of biopsy 60% of the patients did not receive any steroids to treat acute GvHD.
Chronic GvHD. Among 66 patients at risk (alive beyond day 100 without relapse), 37 patients (56%) developed chronic GvHD; it was limited in 14 patients and extensive in 23. In 17 patients who had biopsy after day 100, 11 developed chronic GvHD; it was limited in one patient and extensive in 10. Median time to chronic GvHD was 256 ± 44 days.
Endoscopic and pathologic findings
Histologic digestive GvHD was found in 71 of 95 (73.1%) patients. Pathologic grade was as follows: 33 (34.7%) patients had grade I, 23 (24.2%) patients had grade II, 12 (12.6%) patients had grade III, and 3 (3.2%) patients had grade IV histologic digestive GvHD. Among patients with no pathologic evidence of GvHD (n = 24), drug-induced GI tract symptoms was the probable cause in 21, whereas proven viral infection was found in only 3 patients.
Quantitative assessment
Data on pathologic features among the 95 patients who had biopsies are summarized in Table 3. Among 95 patients who underwent endoscopy, the appearance of the stomach and of the duodenum was normal in 75%. Minor changes, such as erythema, were found in 21%, whereas major abnormalities such as edematous appearance of the mucosa and ulcers were seen in 4% of the cases. If the analysis was restricted to the 65 patients with acute GvHD the findings were the following: normal appearance 57%, minor changes 38%, and major abnormalities 8%. The correlation between what was seen by the endoscopist and the histologic findings is summarized in Table 4. Of note, the gross appearance of the upper GI tract and the pathologic findings did not correlate with the diagnosis of pathologic GvHD, but did correlate significantly to numerous parameters related to the inflammatory process. The sensitivity and specificity of each marker in relation to clinical GvHD are summarized in Table 5.
Qualitative aspects
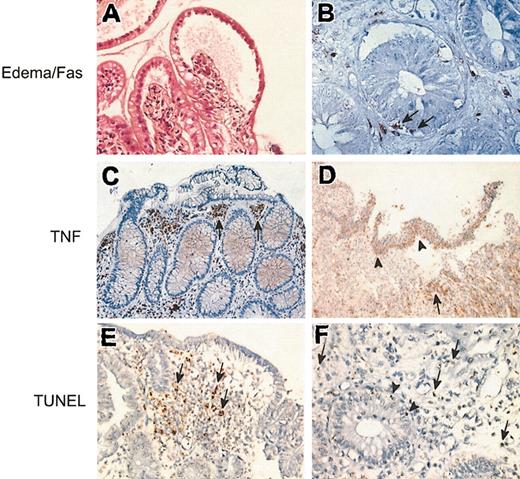
When present, the edema was preferentially distributed in the upper part of the duodenal villi (Figure 1A,E), accumulating under the split superficial epithelium in cases of severe GvHD (Figure 1A). Eosinophils, when present, were also preferentially located in the upper part of the duodenal villi, often associated with edema. Most of them were degranulated, as observed on May-Grünwald-Giemsa stains, and under the electron microscope. The recruitment and degranulation of eosinophils is a sign of in situ activation of these cells, as already shown in a previous study.19 Neutrophils, as mononuclear cells stained with CD45 and CD68, had no specific distribution; they were found in the full thickness of the lamina propria. Few of them were found in the glandular epithelium. On the contrary, cells stained with the antibody directed against TNF (as against the 2 types of TNFRs) were preferentially located in the upper part of the lamina propria, often grouped in small amounts (Figure 1C). They were not found in the epithelium, except in cases of severe GvHD, with a split of the superficial epithelium, where a massive infiltration of inflammatory cells expressing TNF was observed (Figure 1D). Cells expressing Fas were not numerous, but present only in biopsies with proven pathologic GvHD. These inflammatory cells were located both in the lamina propria and in the glandular epithelium (Figure 1B). The TUNEL assay showed that apoptotic cells could be found in the lamina propria (Figure 1E), but also in the glandular epithelium (Figure 1F) in cases of severe digestive GvHD. Ultrastructural analysis of the apoptotic cells showed that surrounding macrophages phagocytosed some of them.
Pathologic study of GvHD in the digestive tract. (A) The superficial epithelium of a villous is split from the lamina propria, with a large area of subepithelial edema. Hematoxylin-eosin, original magnification × 400. (B) Immunostaining with an antibody directed against CD95. Cells located in the glandular epithelium (arrows) as cells located in the lamina propria are stained. Indirect immunoperoxydase, original magnification × 800. (C-D) Immunostaining with an antibody directed against TNF. (C) Numerous stained cells are found in the upper part of the lamina propria (arrows). Indirect immunoperoxidase, original magnification × 250. (D) Full split of the superficial epithelial layer; the cells expressing TNF are mainly located in the split epithelium (arrowheads) or just beneath (arrow). Indirect immunoperoxidase, original magnification × 40. (E-F) TUNEL reactions. (E) Apoptotic bodies (arrows) are mainly located in the upper part of the lamina propria, in close vicinity to the edematous area; original magnification × 250. (F) Apoptotic bodies are mainly found in the lamina propria (arrows), but few apoptotic bodies are also found in the glandular epithelium (arrowheads); original magnification × 600.
Pathologic study of GvHD in the digestive tract. (A) The superficial epithelium of a villous is split from the lamina propria, with a large area of subepithelial edema. Hematoxylin-eosin, original magnification × 400. (B) Immunostaining with an antibody directed against CD95. Cells located in the glandular epithelium (arrows) as cells located in the lamina propria are stained. Indirect immunoperoxydase, original magnification × 800. (C-D) Immunostaining with an antibody directed against TNF. (C) Numerous stained cells are found in the upper part of the lamina propria (arrows). Indirect immunoperoxidase, original magnification × 250. (D) Full split of the superficial epithelial layer; the cells expressing TNF are mainly located in the split epithelium (arrowheads) or just beneath (arrow). Indirect immunoperoxidase, original magnification × 40. (E-F) TUNEL reactions. (E) Apoptotic bodies (arrows) are mainly located in the upper part of the lamina propria, in close vicinity to the edematous area; original magnification × 250. (F) Apoptotic bodies are mainly found in the lamina propria (arrows), but few apoptotic bodies are also found in the glandular epithelium (arrowheads); original magnification × 600.
TRM and its relationship to pathologic findings
TRM at day 90. By univariate Cox analysis, more than 20 neutrophils per field (P = .0025), more than 5 apoptotic cells per field within the cellular infiltrate (P < .0001), and more than 5 Fas-stained cells per field within the cellular infiltrate (P = .0006) were significantly associated with increased TRM at day 90. By multivariate analysis, including pathologic factors only, more than 5 apoptotic cells per field within the cellular infiltrate (P < .0001) and more than 20 neutrophils per field (P = .0003) remained independent risk factors with relative risk (RR) of death of 24.0 (95% CI, 8.3-69.5) and 7.5 (95% CI, 2.1-27), respectively. When adding clinical GvHD factors to the model, more than 5 apoptotic bodies within the infiltrate remained significant (P < .0001) and stage II or more acute GvHD of the liver was the only other significant factor (P < .0001; Table 6). Of note, the models even with a P value of .05 could select none of the other classical clinical parameters such as age or unrelated transplant.
TRM at day 365. By univariate Cox analysis, numerous risk factors reached statistical significance. These included presence (RR = 4.1, P < .0001) and overall severity of GvHD on gut biopsy (pathologic grade II or higher; RR = 3.2, P < .0001), edema (RR = 3.6, P < .0001), and characteristics of the cellular infiltrate (> 10 eosinophils/field [RR = 7.7, P = .0002], > 10 neutrophils/field [RR = 11.7, P < .0001], > 40 lymphocytes/field [RR = 7.7, P < .0001], and > 20 macrophages/field [RR = 3.4, P = .0007]). The extent of apoptosis on gut biopsy reached significance also both in epithelial cells and within the cellular infiltrate (presence and > 5 apoptotic epithelial cells [RR = 4.0, P = .0001 and 2.7, P = .0004, respectively], and presence and > 5 apoptotic cells within the infiltrate [RR = 4.9, P < .0001 and 27.2, P < .0001, respectively]). Finally, in univariate analysis, Fas, TNF, and TNFR expression were associated with worse outcome at 1 year (P < .0001 for all variables; Fas; yes and > 5 cells/field [RR = 3.4 and 4.0, respectively]; TNF, yes and > 20 cells/field [RR = 9.2 and 3.6, respectively]; and TNFRs, yes [RR = 3.2 and 3.3 for TNFR-1 and TNFR-2, respectively]). Among clinical factors, stage II or more liver (RR = 4.9) and gut (RR = 4.4) acute GvHD, chronic GvHD and its extensive form (RR = 9.1 and 7.6, respectively), also reached significance (P < .0001) in univariate analysis.
By multivariate analysis, including only pathologic factors, the only selected factor was the number of apoptotic cells within the cellular infiltrate, any (P < .0001) or more than 5/field (P < .0001), with relative risk of death of 4.1 (95% CI, 2.2-7.5) and 12.2 (95% CI, 4.5-32.6), respectively. By multivariate analysis, adding both clinical and pathologic risk factors, 3 factors remained significantly associated with a worse outcome at 1 year: more than 5 apoptotic cells within the cellular infiltrate (P < .0001), stage II or higher acute liver disease (P < .0001), and chronic GvHD (P < .0001; Table 6). No other clinical factors could be selected, even when assigning a classical P value of .05.
Discussion
In this prospective study, we show that, in humans, both TNF-α and Fas are expressed in the GI tract during GvHD, and that the presence of neutrophils within the cellular infiltrate is highly predictive of TRM.
In GvHD, apoptosis of epithelial cells is the hallmark of GvHD and is the final event of the allogeneic reaction. Part of the apoptotic cells within the cellular infiltrate stem from dead epithelial cells that will be ingested by macrophages from the infiltrate. The phagocytosis of this epithelial debris represents a way of clearing dead cells when the inflammatory process is intense. It is therefore logical that apoptotic epithelial cells and apoptotic bodies within the cellular infiltrate were both highly sensitive and specific markers of GvHD of the GI tract.
We studied the expression of 2 main mediators of apoptosis in GvHD: TNF-α and Fas. Both were expressed in situ in biopsies of patients with digestive GvHD. Although extensive data support the role of TNF-α and Fas in rodent models, data on in situ expression of these molecules in humans are lacking. The role of TNF-α in the genesis of GvHD has been studied largely in rodent models,20-24 and its role in human is suggested by several studies.25-31 This was first suggested in mice injected with anti–TNF-α32 and subsequently supported by numerous experimental studies in rodent models.33-42 The role of TNF-α in human GvHD was suggested by some benefit of a monoclonal anti–TNF-α antibody in patients with severe refractory GvHD43 or when used as GvHD prophylaxis during the pretransplantation conditioning.27,44 However, to the best of our knowledge, direct evidence of in situ expression of TNF and of its receptors in human GvHD in a large cohort of patients has never been studied. Here we show that TNF-α was expressed in all (100%) biopsies of patients with acute GvHD. More importantly, quantitative estimates suggest that the number of cells per field expressing TNF was highly specific of acute GvHD. However, TNF sensitivity was rather low. This latter result supports experimental studies showing nonspecific TNF-α release in processes not directly related to GvHD, in particular to secondary effect of irradiation-induced damage of epithelial cells. TNFRs (R1 and R2) were both expressed in GI biopsies, thus suggesting a functional pathway, but none reached significant sensitivity and specificity with regard to pathologic diagnosis of GvHD. The role of Fas-mediated apoptosis has also been evidenced in GvHD models.42,45-47 When we designed this study we were particularly interested by the findings of Hattori et al.45 These authors examined the effects of neutralizing anti-Fas ligand (Fas-L) and anti–TNF-α MoAb in a lethal acute GvHD model in mice. Whereas the treatment with either anti–Fas-L or anti–TNF-α MoAb alone significantly delayed the mortality and improved body weight, complete protection was achieved by the administration of both MoAbs. Pathologic examination indicated differential effects of anti–Fas-L or anti–TNF-α MoAb on GvHD-associated pathologies. Hepatic lesions were improved by anti–Fas-L but not by anti–TNF-α MoAb. In contrast, intestinal lesions were improved by anti–TNF-α but not anti–Fas-L MoAb. Thus, these results indicate that Fas-L and TNF-α differentially contribute to GvHD pathologies. Our own recent findings, in an experimental model of lymphocyte transfer in severe combined immunodeficient (SCID) mice, also strongly support a role of the Fas/Fas-L pathway in mice GvHD.48 However, to the best of our knowledge the role of this pathway in human GvHD has never been directly assessed. We did find that Fas was expressed in GvHD, thus suggesting a pathologic role of this pathway in humans. However, Fas expression within the cellular infiltrate failed to reach the stringent criteria we stated for statistical significance for both sensitivity and specificity. Whether this significance could be reached by the study of additional samples would need further studies and confirmatory results from other groups.
We also aimed to characterize, in situ, the number of lymphocytes and of inflammatory cells within the lamina propria. Cell counts were based on an individually defined mucosal tissue unit. Edema intensity was also assessed. Lymphocyte numbers (> 20/field) and edema intensity, both reflecting acute GvHD severity and intensity of the inflammatory reactions, were both sensitive and specific of GvHD. More interesting, in our opinion, was the association, by multivariate analysis of pathologic factors, of increased early TRM with the presence of more than 20 neutrophils per field. We recently pointed out that activated eosinophils are associated with severe pathologic forms of digestive GvHD,19 and we have already reported the involvement of macrophages, the main producers of TNF, in lethal forms of GvHD.49 All these studies strongly suggest a deleterious effect of cells of the innate immunity in human GvHD. Because these cells are attracted to damaged tissues by soluble factors including chemokines, it could be worthwhile to look for the in situ expression of these molecules or of genetic polymorphisms associated with increased chemokine production in human GvHD. We previously reported that IL-5, the major mediator that recruits, activates, and prolongs eosinophils survival is expressed in human digestive GvHD.19 Experimental studies in rodents suggest that among chemokines, macrophage inflammatory protein 1α (MIP-1α) is critical to the recruitment of CCR5+CD8+ T cells during GvHD.50,51 This aspect of chemokine-mediated effector cell recruitment and antigen nonspecific damage induced by neutrophils and eosinophils is worthwhile to explore in humans given recent results in rodents suggesting that acute GvHD does not require allogeneic antigen expression on host epithelium.52,53 Recently, it was also demonstrated that T-cell expansion occurred early and contracted back to baseline by day 14, before the onset of GvHD mortality.9,10 Three weeks after transplantation, target organs are infiltrated by cells of the myeloid lineage, suggesting an important role for non-T cells as GvHD effectors. In humans, even “early” biopsies are performed at this late stage of the process, and the present study showing a deleterious role of neutrophils is also in favor of an important role for non-T cells in human GvHD.
Finally, stage II or higher acute and chronic GvHD of the liver were other factors of increased TRM. It is well recognized that chronic GvHD is the main determinant of long-term outcome after allogeneic transplantation and is thus logically associated with increased 1-year nonrelapse mortality. Stage II or higher acute liver disease was less expected. However, it should be recalled that our patient population was not a random one. We did perform biopsies for clinical signs suggestive of GvHD and indeed more than 85% of these patients did have GvHD. Thus, what this latter finding shows is that among patients with gut GvHD, stage II or higher liver disease has a bad prognostic value, a finding reminiscent of systems grading GvHD severity. However, grade itself was not associated with transplant mortality in this study. It is obvious that this would need confirmatory results from other groups. Nevertheless, this would suggest that GvHD intensity as assessed by the presence of apoptotic cells within the infiltrate is a more powerful factor than classical grading system or that using an objective way to measure the intensity of the process is a more reliable tool than assessing stool volume. Other authors, including the Seattle group,54 already pointed out the limits of the classical way we assess GvHD severity through grading systems. During the time period of this study 256 patients underwent allogeneic SCT in our group. The 95 patients who underwent upper digestive tract biopsies (37% of the total population) had significantly more GvHD (and more digestive GvHD) than patients who did not undergo biopsy. They represent more than 95% of the patients with digestive symptoms as initial, or recurring, symptoms of GvHD (data not shown). However, one unavoidable bias of this study is that we performed upper digestive tract biopsies in patients with symptoms. Thus, whereas our estimates of sensitivity are correct, estimates of specificity are more questionable because true specificity would be the estimate of no pathologic sign in patients without clinical symptoms. For obvious ethical reasons we did not perform such a study.
In conclusion, in this prospective clinical study we showed that the number of apoptotic cells within the cellular infiltrate was a major factor of TRM. The role of cells of the innate immunity, particularly that of neutrophils, should be studied in experimental models.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-03-0909.