Abstract
Activating mutations of FMS-like tyrosine kinase 3 (FLT3) are present in approximately 30% of patients with de novo acute myeloid leukemia (AML) and are associated with lower cure rates from standard chemotherapy-based treatment. Targeting the mutation by inhibiting the tyrosine kinase activity of FLT3 is cytotoxic to cell lines and primary AML cells harboring FLT3 mutations. Successful FLT3 inhibition can also improve survival in mouse models of FLT3-activated leukemia. CEP-701 is an orally available, novel, receptor tyrosine kinase inhibitor that selectively inhibits FLT3 autophosphorylation. We undertook a phase 1/2 trial to determine the in vivo hematologic effects of single-agent CEP-701 as salvage treatment for patients with refractory, relapsed, or poor-risk AML expressing FLT3-activating mutations. Fourteen heavily pretreated AML patients were treated with CEP-701 at an initial dose of 60 mg orally twice daily. CEP-701–related toxicities were minimal. Five patients had clinical evidence of biologic activity and measurable clinical response, including significant reductions in bone marrow and peripheral blood blasts. Laboratory data confirmed that clinical responses correlated with sustained FLT3 inhibition to CEP-701. Our results show that FLT3 inhibition is associated with clinical activity in AML patients harboring FLT3-activating mutations and indicate that CEP-701 holds promise as a novel, molecularly targeted therapy for this disease.
Introduction
Acute myeloid leukemia (AML) is an aggressive malignancy that results in progressive bone marrow failure. Despite the success of initial treatments, most patients with AML eventually die of their disease. Advances in understanding the molecular and biologic underpinnings of AML have provided targets for new therapeutic agents. Unfortunately, these have yet to be translated into better patient outcomes. FMS-like tyrosine kinase 3 (FLT3), a receptor tyrosine kinase expressed by immature hematopoietic cells, is important in the development of stem/progenitor cells and in the immune system.1-3 The wild-type FLT3 receptor is highly expressed in many types of leukemia.4-6 FLT3 gene mutations are present in the leukemic blasts of approximately one third of patients with AML. Lower incidences of mutations are observed in patients with myelodysplastic syndrome (MDS) and acute lymphoblastic leukemia.7-13 Internal tandem duplication (ITD) mutations are localized to the juxtamembrane region and are expressed in 17% to 34% of patients with AML. Another 7% of leukemias have point mutations in the activation loop of the kinase domain.14,15 Taken together, mutations of FLT3 are the most prevalent genetic alteration in AML. Both major types of activating FLT3 mutations identified in AML result in constitutive FLT3 activity.14,16
Studies indicate that constitutively activated FLT3 may play a significant role in leukemogenesis.17,18 Constitutively activated forms of FLT3 transform hematopoietic cell lines,19-23 rendering their growth independent of cytokines and causing leukemia when the transformed cells are injected into syngeneic mice. Transgenic mice and retrovirally infected mouse bone marrow expressing activated FLT3 develop myeloproliferative syndromes.24,25 When expressed in transgenic mice already expressing promyelocytic leukemia–retinoic acid receptor α (PML-RARα), FLT3 mutations cooperate to result in promyelocytic leukemia with high penetrance and shortened lag times.26 The presence of FLT3/ITD mutations in patients withAML is associated with lower cure rates when these patients are treated with standard chemotherapy-based regimens.8-11,13,27,28
In vitro work has shown that targeting the mutation by inhibiting the tyrosine kinase activity of FLT3 is cytotoxic to cell lines and to primary AML cells harboring FLT3 mutations.22,23,29-36 Successful FLT3 inhibition also improves survival in mouse models of FLT3-activated leukemia.23,35,36
CEP-701 (Cephalon, West Chester, PA), a chemically synthesized derivative of K-252a and a fermentation product of Nonomurea longicatena, belongs to a class of compounds identified as indolocarbazole alkaloids.37 Preclinical studies have characterized this orally available compound as a potent FLT3 inhibitor with an in vitro IC50 of 2 to 3 nM.23 CEP-701 displays low nanomolar inhibition of TrkA and vascular endothelial growth factor receptor (VEGFR) kinases, but it is not a potent inhibitor of other receptor tyrosine kinases related to FLT3. IC50s greater than 500 nM against KIT, FMS, and platelet-derived growth factor receptor β (PDGF-Rβ) CEP-701 is cytotoxic to several FLT3-expressing human leukemia–derived cell lines and to primary bone marrow–derived AML blasts harboring FLT3 mutations.23 The drug also prolongs survival in a mouse model of FLT3/ITD leukemia.23 Previous studies showed the drug to have a low toxicity profile in animals. Pharmacokinetic studies after single doses of oral CEP-701 administered to healthy volunteers showed area under the curve (AUC) values ranging from 929 to 7889 ng/h·mL and a drug half-life between 6.8 and 9.2 hours. CEP-701 was further studied for safety and tolerability in patients with cancer (prostate cancer, lymphoma, and advanced, incurable solid malignancies) and showed pharmacokinetic profiles similar to those of healthy volunteers.
We undertook a limited phase 1/2 clinical trial to determine the in vivo hematologic effects of single-agent CEP-701 as salvage treatment for patients with refractory, relapsed, or poor-risk AML expressing FLT3-activating mutations. Based on the in vitro data, we hypothesized that inhibiting FLT3 in vivo would result in clinical activity against FLT3 mutation–positive AML. We also conducted studies to determine the extent of FLT3 inhibition in vivo by CEP-701 and whether such inhibition correlated with clinical effects.
Patients and methods
Patient selection
Patients were eligible if they had refractory, relapsed, or poor-risk AML; were older than 18 years; had Eastern Cooperative Oncology Group (ECOG) performance status less than 3; had recovered from the adverse effects of previous therapies and had been off previous treatments for at least 4 weeks; had white blood cell (WBC) counts lower than 30 × 109/L for 7 days immediately before CEP-701 initiation; had adequate renal function (estimated creatinine clearance greater than 60 mL/min) and hepatic parameters (total bilirubin less than 2 times the upper limit of normal and alanine aminotransferase or aspartate aminotransferase less than 2 times the upper limit of normal); and their leukemia tested positive for an activating mutation of FLT3. The diagnosis of AML was confirmed by pathology review at the treating institution, and disease was classified as relapsed or refractory based on the response to the most recent salvage attempt. Patients were required to have evidence of active disease at the time of enrollment and subsequent treatment. Patients who were otherwise medically eligible for enrollment but had WBC counts higher than 30 × 109/L were allowed treatment with hydroxyurea to stabilize the WBC count for up to the first 21 days of therapy (hydroxyurea was discontinued for WBC counts lower than 10 × 109/L). Written informed consent was obtained from all patients according to the Declaration of Helsinki and as approved by the Western institutional review board; some patients agreed to the collection of additional blood or marrow specimens solely for research purposes through a second informed consent form.
Study design
This was a limited phase 1/2, open-label trial of single-agent CEP-701. The primary study end point was to determine the response rate of patients with refractory, relapsed, or poor-risk AML expressing FLT3-activating mutations when administered CEP-701. Secondary end points were to assess safety and tolerability and to estimate the pharmacokinetic and pharmacodynamic effects of CEP-701 in this population. Patients were initially treated at a dose of 40 mg orally twice a day. Dose escalation was planned to 60 mg orally twice a day at the completion of 28 days of treatment for patients without evidence of drug-related toxicity. Patient response assessments were planned based on the completion of two 28-day treatment cycles. Patients with evidence of clinical activity (complete remission [CR] or hematologic response [HR]) continued monthly cycles of CEP-701 until disease progression or evidence of dose-limiting toxicity (DLT). Patients were removed from the study at the completion of 2 planned cycles of CEP-701 if there was no evidence of clinical response, if they had unresolved DLT at any time during the planned treatment course, or at their own request. Following observations that in vivo inhibition of FLT3 was variable in the first 3 patients, the protocol was amended to increase the starting dose to 60 mg twice a day by mouth for all subsequent patients, with a planned dose escalation to 80 mg orally twice a day at the completion of 28 days of treatment.
Assessment of toxicity and response
Patients were monitored at least twice weekly for the duration of the study. Measurements included hematologic tests, biochemical tests, physical examination, safety assessments for adverse effects, and observations of vital signs. Toxicity was graded according to the Common Toxicity Criteria, version 2.0, of the National Cancer Institute (Cancer Therapy Evaluation Program, May 1998, http://ctep.cancer.gov/reporting/CTC-3.html). Response was determined after the first 28-day treatment period and monthly thereafter. CR was defined as a cellular bone marrow with 5% or fewer leukemic blasts and normalization of peripheral blood cell counts (granulocyte count greater than 109/L; platelet count greater than 100 × 109/L). HR was defined as a greater than 50% reduction in the absolute number of peripheral blood blasts (determined by routine peripheral blood differential) or at least a 50% reduction in the percentage of bone marrow blasts (determined by flow cytometry). Pathologic reviews were performed by hematopathologists at the treating centers who were blinded to patient status.
Pharmacokinetics and determination of CEP-701 plasma concentrations
Samples for pharmacokinetic analysis were collected immediately before and 1 hour after the morning dose of CEP-701 on treatment days 1 and 28. A 12-hour trough level was also measured before the morning dose of CEP-701 on day 2. Trough levels were obtained from several patients at additional time points for correlative studies.
Concentrations of CEP-701 in human plasma samples were determined using a validated high-performance liquid chromatography method with fluorescence detection. The method involved liquid-liquid extraction of CEP-701 from 0.1 mL human plasma into a mixture of ethyl acetate/methylene chloride in a 4:1 (vol/vol) proportion, followed by reverse-phase chromatography on a Hypersil BDS phenyl column (P. Robertson, personal oral communication, November 2003).
Determination of FLT3 mutations
Patient peripheral blood or bone marrow samples were tested to confirm the presence of FLT3-activating mutations. Briefly, DNA was extracted directly from blood or bone marrow samples using the QIAamp DNA kit (Qiagen, Valencia, CA) according the manufacturer's instructions. ITD presence and kinase domain point (D835) mutations were detected through multiplex polymerase chain reaction (PCR) and EcoRV digestion and then by capillary electrophoresis, as described previously.38 A specimen was considered to harbor an FLT3 mutation if the mutation was present at a ratio greater than or equal to 5% of total FLT3 signal (mutant or wild type). All FLT3 mutations were confirmed by DNA sequencing. Sequences were analyzed and allelic ratios calculated using Sequencher software (Gene Codes, Ann Arbor, MI).
In vitro cytotoxicity assay
Marrow aspirates were obtained from the posterior iliac crest of all trial patients before initiating treatment with CEP-701. Marrow mononuclear cells were isolated using Ficoll-Hypaque (Amersham, Piscataway, NJ) centrifugation and were cultured in RPMI/10% fetal bovine serum (FBS) with antibiotics (penicillin and streptomycin) or were stored viably frozen in FBS/10% dimethyl sulfoxide (DMSO). Blast purity was typically greater than 90% in the post-Ficoll product. MTT and Annexin V assays were performed as described previously on all samples for which adequate cells were procured.29
Assay for FLT3 autophosphorylation in vivo
Peripheral blood was collected in heparinized tubes and promptly chilled on ice. Samples were centrifuged for 10 minutes at 900g at 4° C. Plasma was removed and stored frozen at –80° C. The buffy coat was carefully transferred to ice-cold phosphate buffered saline (PBS), layered onto chilled Ficoll-Hypaque, and centrifuged for 5 minutes at 600g at 4° C. All subsequent steps were carried out at 4° C. Mononuclear cells were collected and washed rapidly 3 times in red blood cell lysis buffer (0.155 M NH4Cl, 0.01 M KHCO3, 0.1 mM EDTA [ethylenediaminetetraacetic acid]), then washed once in PBS. Cells were lysed by resuspension in lysis buffer (20 mM Tris, pH 7.4, 100 mM NaCl, 1% Igepal [Sigma, St Louis, MO], 1 mM EDTA, 2 mM NaVO4) plus complete protease inhibitors (Complete; Roche, Indianapolis, IN) for 30 minutes while rocking. The extract was clarified by centrifugation at 14 000 rpm, and the supernatant was assayed for protein (Bio-Rad, Richmond, CA). Anti-FLT3 antibody (S18; Santa Cruz Biotechnology, Santa Cruz, CA) was added to the extract for overnight incubation, and protein A Sepharose (Upstate Biotechnology, Lake Placid, NY) was added for 2 hours. After sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transfer to polyvinylidene difluoride (PVDF) membranes (Immobilon; Millipore, Bedford, MA), immunoblotting was performed with antiphosphotyrosine antibody (4G10; Upstate Biotechnology) to detect phosphorylated FLT3, and the extract was stripped and reprobed with anti-FLT3 antibody to measure total FLT3 protein. Proteins were visualized using enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ), and resultant films were scanned using an AGFA Arcus 1200 laser scanner (Morstel, Belgium). Densitometry was performed with the public domain National Institutes of Health Image program (available at http://rsb.info.nih.gov/nih-image/).
Ex vivo bioassay: TF/ITD cell line development
TF-1 cells (human AML M6, lacking protein expression of FLT3; ATCC, Rockville, MD) were transfected by electroporation with a vector (pCIneo; Promega, Madison, WI) containing the cDNA for an FLT3/ITD mutation isolated from an AML patient.22 After selection with G418, a clonal line (designated TF/ITD) expressing constitutively phosphorylated FLT3 was obtained through limiting dilution. TF/ITD cells are growth factor independent and are cultured in RPMI/10% FBS.
Ex vivo bioassay: assay of FLT3 autophosphorylation inhibition
To determine the bioactivity of plasma from patients treated with CEP-701, frozen plasma samples were thawed and clarified by centrifugation at 14 000 rpm for 2 minutes. There was no difference in results when fresh plasma was used in comparison with previously frozen plasma (data not shown). For each time point, 4 × 106 TF/ITD cells were incubated with 1 mL plasma at 37° C for 3 hours. Cells were washed once with ice-cold PBS, then lysed and analyzed as described in “Assay for FLT3 autophosphorylation in vivo.”
Results
Enrollment and treatment of patients
From February 2002 to January 2003, 17 patients with relapsed or refractory AML expressing FLT3-activating mutations were enrolled at the 2 study centers. Patients' characteristics are summarized in Table 1. Ten (59%) patients had AML refractory to the most recent salvage chemotherapy; 8 (47%) patients had received 2 or more prior salvage regimens; 3 patients were treated following relapse after allogeneic bone marrow transplantation. The median age was 61 years (range, 18-74 years), and the median ECOG performance score was 1 (range, 0-2).
Three patients were treated with a starting dose of 40 mg CEP-701 orally twice a day (median, 26 days; range, 20-28 days), and 14 patients were treated at 60 mg twice a day (median, 24 days; range, 4-28 days). Three of the latter patients received a subsequent course at 80 mg twice a day (median, 18 days; range, 3-41 days). Eight patients did not complete the initial cycle of treatment (4 because of disease progression, 3 because of death related to infection, and 1 because of persistent nausea and poor oral intake). Twelve patients were treated with concurrent hydroxyurea resulting from elevated WBC counts (median, 9 days; range, 2-21 days). Hydroxyurea doses were adjusted based on the total WBC count. No toxicities were reported related to the combination of the 2 drugs.
Pharmacokinetics
Table 2 lists the plasma concentrations at the noted pharmacokinetic time points. As predicted from preclinical and previous phase 1 studies, the plasma concentrations of CEP-701 varied at each of the time points. Importantly, CEP-701 was rapidly absorbed with quantifiable levels readily observed 1 hour after the initial dose on day 1 at 40 mg (mean, 782.2 ng/mL; range, 510.9-1184.7 ng/mL) and 60 mg (mean, 1604.6 ng/mL; range, 166.5-2649.8 ng/mL). The mean trough level on day 28 from patients receiving 60 mg twice daily dosing was 1925.9 ng/mL (4.4 μM), ranging from 498.2 to 5850.7 ng/mL (1.1-13.3 μM).
Safety profile
CEP-701 was well tolerated; drug-related toxicities were difficult to identify and isolate separately from adverse effects resulting from the patients' underlying leukemia or concurrent medications. Commonly observed toxicities included mild nausea, mild emesis, and mild generalized weakness (not associated with muscle weakness or creatinine kinase elevations) and fatigue. A complete list of the adverse effects possibly attributable to CEP-701 are summarized in Table 3. We observed no increase in the grade or frequency of toxicities associated with the highest doses administered, and no specific drug toxicity resulted in discontinuation of the planned treatment.
Clinical activity
The observed clinical activity of CEP-701 is summarized in Table 1. Of the first 3 patients treated, 1 (patient 102) was unable to consistently tolerate oral medication of any kind and eventually withdrew from the trial after 20 days on CEP-701. The 2 remaining patients tolerated oral administration of CEP-701 without adverse effects, but neither responded clinically. Correlative bioassays (see “Correlative studies”) suggested that inadequate FLT3 inhibition might have accounted for the lack of response in at least 1 of these patients. Based on these findings, the study was amended to increase the starting dose to 60 mg orally twice a day, with a planned escalation to 80 mg orally twice a day starting on day 29 provided that no DLT occurred.
Clinical activity was seen in 5 of the next 14 patients treated at an initial CEP-701 dose of 60 mg twice a day. All 5 of these patients had AML that was refractory to previous chemotherapy treatment. In these patients, single-agent CEP-701 effectively lowered peripheral blood blasts, and some patients had evidence of stabilized normal hematopoiesis. One patient showed a decrease in the percentage of bone marrow blasts to less than 5%, whereas the other 4 patients had peripheral blood responses, with blasts decreasing from a range of 27% to 94% to a range of 1% to 10%. These responses were also accompanied by decreased transfusion requirements for red blood cells and platelets in 2 patients and complete transfusion independence in 1 (patient 104). Absolute neutrophil counts became normalized (more than 1000/mm3) in 3 patients during treatment. All responses were of short duration, ranging from 2 weeks to 3 months. The limited responses in these patients might have been at least partially reflective of their refractory, heavily pretreated disease.
Correlative studies
To understand the basis of the clinical activity (or lack thereof) of CEP-701 in individual patients, we carried out a series of correlative and surrogate assays using plasma and leukemia cell samples from patients enrolled in this trial.
In vitro cytotoxicity assays
In vitro cytotoxic responses were assessed using MTT and Annexin V apoptosis assays.23,29 These assays were performed using mononuclear cells from the pretreatment bone marrow samples of the 7 patients in whom adequate cells were available. Six of the 8 AML blast samples demonstrated cytotoxic response to FLT3 inhibition in a typical dose-response fashion (Figure 1). Two of the samples, from patients 101 and 106, did not display cytotoxic response in vitro to CEP-701, despite successful FLT3 inhibition. This lack of response to FLT3 inhibition has been observed in many leukemia cell lines lacking FLT3 mutations and in a minority of primary blast samples harboring FLT3 mutations.23 From these data, we predicted that patients 101 and 106 would be unlikely to respond clinically to CEP-701 based on intrinsic cellular resistance. Consistent with this prediction, neither patient responded clinically. The in vitro cytotoxic responses of the blast samples from the remaining 6 patients (Figure 1) suggested that a clinical response might be expected in this group if FLT3 was continuously inhibited in vivo to a sufficient degree.
Cytotoxicity assay. Bone marrow mononuclear cells from 8 patients treated with CEP-701 were incubated in vitro (in cell culture medium) with increasing concentrations of CEP-701. Each drug concentration point was performed in triplicate. At 72 hours, metabolic activity was determined using the MTT assay. Results are plotted for each sample as percentage (optical density [OD]) of untreated control. Error bars represent SE.
Cytotoxicity assay. Bone marrow mononuclear cells from 8 patients treated with CEP-701 were incubated in vitro (in cell culture medium) with increasing concentrations of CEP-701. Each drug concentration point was performed in triplicate. At 72 hours, metabolic activity was determined using the MTT assay. Results are plotted for each sample as percentage (optical density [OD]) of untreated control. Error bars represent SE.
Determination of FLT3 phosphorylation status in vivo
Our preclinical data suggested that the ability of CEP-701 to induce leukemia blast apoptosis in vitro was directly related to continuously inhibiting FLT3 autophosphorylation to less than 10% to 15% of baseline activity. Understanding the clinical results noted with CEP-701 treatment required the ability to measure the level of in vivo FLT3 inhibition. Simply measuring the concentration of drug in plasma would be insufficient for this purpose because CEP-701 is highly protein bound, and free drug levels would be expected to vary considerably from patient to patient. We anticipated that measuring in vivo FLT3 autophosphorylation would be difficult in patients with low blast counts before treatment and in patients with initially high blast counts who subsequently responded to CEP-701 during the course of therapy. Therefore, we directly measured FLT3 inhibition in vivo early during therapy with CEP-701 in a number of patients and then used these results to validate a more convenient surrogate assay for in vivo FLT3 inhibition.
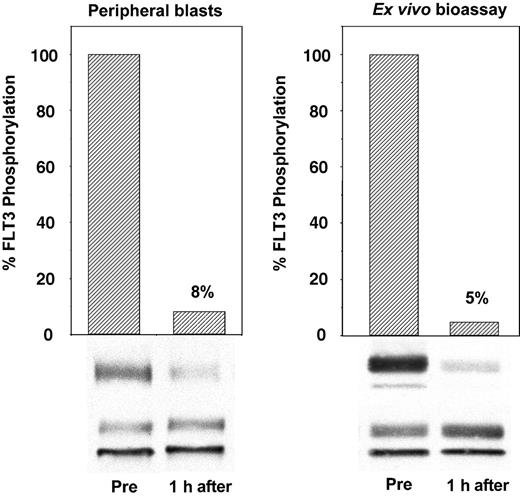
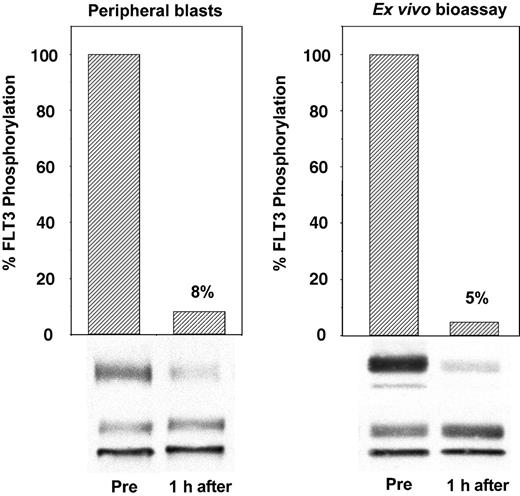
To establish that CEP-701 inhibited the FLT3 receptor within the patients' leukemic blasts, we obtained peripheral blood samples immediately before and 1 hour after the initial dose of drug. Plasma and leukemia cells from these blood samples were quickly separated, and the plasma was frozen for later use. Leukemia cells were lysed and subjected to immunoprecipitation and immunoblotting to directly measure FLT3 autophosphorylation. An example of this is shown in Figure 2 (left), in which a single dose of CEP-701 inhibited FLT3 autophosphorylation to 8% of the pretreatment control. In the surrogate assay, the plasma from these same samples collected before and after the initial dose of CEP-701 was used ex vivo to treat TF/ITD cells expressing an FLT3-activating ITD mutation. The results of this ex vivo bioassay (Figure 2, right) were then compared with the results from the direct assay of FLT3 in the patient's blasts. This comparison was made when peripheral blasts were available throughout the trial to establish that the ex vivo bioassay is a reliable measure of the in vivo FLT3 inhibitory activity of CEP-701. The advantage of the surrogate assay is that is does not rely on the presence of large numbers of circulating blasts. It also allows for a more standardized comparison of this critical laboratory parameter at any point during treatment with CEP-701 without the variables induced in the direct assay by differences in the time from the blood draw to blast cell isolation to cell lysis.
Ex vivo bioassay for FLT3 inhibition. The immunoblot on the left was derived from peripheral blood leukemia cells isolated from a patient before and 1 hour after the initial dose of CEP-701. The blot on the right was obtained from TF/ITD cells exposed to the plasma from the same patient at the same time point. FLT3 was immunoprecipitated from detergent extracts of leukemia cells, resolved with SDS-PAGE, and transferred to PVDF membranes. The phosphorylated state of the receptor (upper bands, P-FLT3) was determined by immunoblotting with an antiphosphotyrosine antibody. Membranes were then stripped and reprobed with anti-FLT3 antibody to assess total FLT3 protein (lower bands, FLT3). Densitometric analysis of phosphorylated FLT3, normalized for the amount of total FLT3, is displayed graphically with columns.
Ex vivo bioassay for FLT3 inhibition. The immunoblot on the left was derived from peripheral blood leukemia cells isolated from a patient before and 1 hour after the initial dose of CEP-701. The blot on the right was obtained from TF/ITD cells exposed to the plasma from the same patient at the same time point. FLT3 was immunoprecipitated from detergent extracts of leukemia cells, resolved with SDS-PAGE, and transferred to PVDF membranes. The phosphorylated state of the receptor (upper bands, P-FLT3) was determined by immunoblotting with an antiphosphotyrosine antibody. Membranes were then stripped and reprobed with anti-FLT3 antibody to assess total FLT3 protein (lower bands, FLT3). Densitometric analysis of phosphorylated FLT3, normalized for the amount of total FLT3, is displayed graphically with columns.
Determination of the allelic ratio of FLT3 mutant to wild type
A high ratio of FLT3 mutant to wild type has been shown to have prognostic significance in patients with AML harboring FLT3 mutations.27 We were interested in evaluating this parameter to identify any potential correlation with clinical response to FLT3 inhibition. PCR determination of genomic DNA allelic ratios was measured before the initiation of treatment with CEP-701 in all patients treated at the Sidney Kimmel Cancer Center at Johns Hopkins University.
Correlation of bioassay and clinical activity
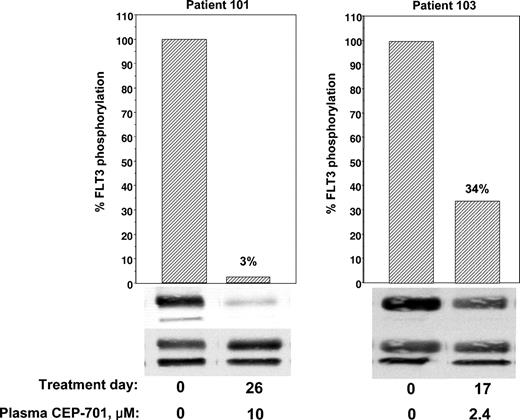
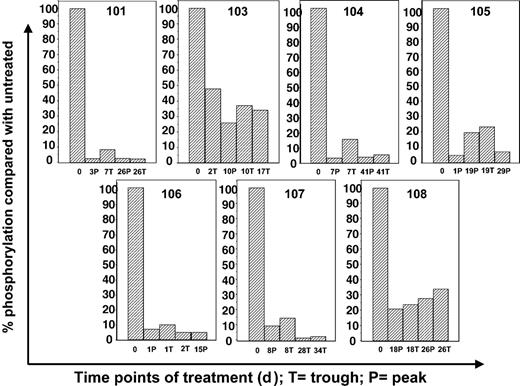
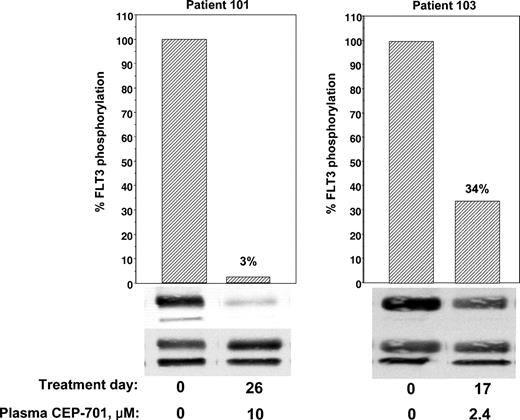
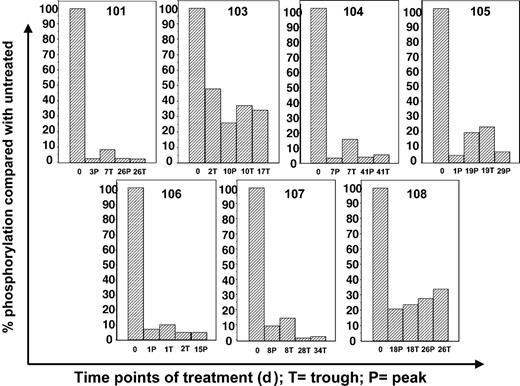
Correlative data from 2 of the first 3 patients (patients 101 and 103) treated at 40 mg orally twice a day was analyzed before continuing with the trial. Patient 102 had difficulty tolerating oral medications of any kind because of persistent nausea and emesis that predated her enrollment in the trial by several weeks. Despite aggressive use of antiemetics, her ability to ingest CEP-701 was inconsistent, and she eventually withdrew from the trial. Patients 101 and 103 had no difficulty taking their oral medications and CEP-701, but neither patient responded clinically. Initial marrow blasts of all 3 patients were studied for in vitro responsiveness to CEP-701; blasts from patient 101 failed to show a significant in vitro cytotoxic response to the drug (Figure 1). We next used ex vivo bioassay to measure the FLT3 inhibitory activity of plasma from these patients. As shown in Figure 3, patient 101 achieved a trough level (10 μM) of the drug sufficient to inhibit FLT3 autophosphorylation to 3% of the pretreatment control. In patient 103, FLT3 was only inhibited to 34% at trough levels (2.4 μM), well above the goal of 10% to 15%. This correlation provided a possible explanation for the lack of clinical activity in the initial patients. Leukemia in patient 101 was intrinsically resistant to CEP-701, whereas Patient 103, whose disease was sensitive in vitro, failed to achieve adequate plasma levels of the free drug. Hence, the clinical trial was amended to allow a starting dose of CEP-701 of 60 mg orally twice per day, and clinical activity was seen in 5 of the next 14 patients. Correlation of the clinical activity and the results of the ex vivo bioassay for FLT3 inhibition provided important information in the 8 patients tested. Six patients had leukemia cells that responded to CEP-701 in the in vitro cytotoxicity assay (Figure 1), and 3 of these patients (patients 104, 105, and 107) achieved more than 85% sustained inhibition of FLT3 blast activity, as measured by the ex vivo bioassay (Figure 4). All 3 of these patients had clinical responses to CEP-701. In contrast, the 2 patients with blasts that responded to CEP-701 in vitro, but who failed to achieve sustained inhibition of FLT3 greater than 85% (patients 103 and 108), did not respond clinically to CEP-701. Overall, the targeted level of FLT3 inhibition of 10% to 15% of baseline activity was achieved in 8 (1 treated at 40 mg and 7 treated at 60 mg) of 14 patients from whom adequate plasma samples were available. These results are summarized in Table 1.
Correlative data on patients 101 and 103. Trough plasma samples from patients 101 and 103 were used for ex vivo bioassays of FLT3 phosphorylation (performed as described in Figure 2).
Correlative data on patients 101 and 103. Trough plasma samples from patients 101 and 103 were used for ex vivo bioassays of FLT3 phosphorylation (performed as described in Figure 2).
Correlative data using ex vivo bioassay for treated patients. Plasma samples from patients 101 and 103 to 108 were obtained at the treatment time points noted. Ex vivo bioassay was performed as described above for Figure 2. Results for patients 101 and 103 were based on 40 mg twice-a-day dosing. All other results were from patients treated at 60 mg twice a day, with bars representing data from 80 mg twice-a-day dosing. P indicates peak (1 hour after CEP-701 dose); T, trough (12 hours after dose); number, days on therapy.
Correlative data using ex vivo bioassay for treated patients. Plasma samples from patients 101 and 103 to 108 were obtained at the treatment time points noted. Ex vivo bioassay was performed as described above for Figure 2. Results for patients 101 and 103 were based on 40 mg twice-a-day dosing. All other results were from patients treated at 60 mg twice a day, with bars representing data from 80 mg twice-a-day dosing. P indicates peak (1 hour after CEP-701 dose); T, trough (12 hours after dose); number, days on therapy.
As an example of a typical responding patient, patient 107, a 69-year-old woman with AML that was refractory to high-dose Ara-C therapy, entered the trial with a rapidly rising WBC count of 15 200/mm3, 85% of which were blasts. She received CEP-701 at 60 mg twice daily, without concomitant hydroxyurea. By day 8, and continuing through day 12, her WBC count had fallen to 1470/mm3, with 6% blasts. This correlated with FLT3 inhibition to 10% of baseline, as measured in the ex vivo bioassay. By day 20, however, the WBC count rose again to 21 990/mm3 (75% blasts), and the surrogate assay showed that FLT3 was suppressed to only 19% at peak time points. Hydroxyurea was administered orally for 2 days to keep the WBC count below 20 000/mm3. Bone marrow biopsy on day 28 revealed no decrease in blast percentage compared with that on day 1. On day 29, she received a CEP-701 dose increase to 80 mg twice daily, and her WBC count again fell precipitously to 750/mm3, with 6% blasts. The ex vivo bioassay revealed FLT3 to be inhibited to 2% to 3% of baseline activity at this dose. She had a persistent fungal infection and elected to enter hospice on day 35. Her WBC count was now 1130/mm3, with 0% blasts. No bone marrow was obtained at this time.
The individual allelic ratios of FLT3 mutant to wild type determined before treatment with CEP-701 did not appear to predict response in the small number of patients tested (data not shown). However, PCR data from patient 105 provided an interesting correlation between a changing allelic ratio and the clinical response. At the time of enrollment, patient 105 (who had an FLT3/ITD mutation) had a WBC of 3500/mm3 and a ratio of mutant FLT3 to wild type of 6.5. After 14 days of treatment with CEP-701, the WBC decreased to 980/mm3, and the FLT3 allelic ratio decreased to 0.6. Unfortunately, the initial response in patient 105 was transient with a day 28 WBC of 37 000/mm3 and a day 49 WBC of 48 000/mm3. The FLT3 allelic ratio appeared to correlate with disease progression, increasing to 6.4 (day 28) and further to 17.5 (day 49).
Discussion
This study is the first demonstration of the clinical and biologic effects of an agent specifically targeting FLT3 mutations in patients with AML. CEP-701 was a well-tolerated and convenient oral medication that showed clinical activity in a group of older and heavily pretreated patients, most of whom had refractory AML. These patients were uniformly poor-risk candidates based on age, number of previous salvage treatments, poor response to the most recent chemotherapy treatments, and the presence of FLT3-activating mutations. Despite these high-risk features, most patients tolerated outpatient treatment, and the toxicities seen with CEP-701 therapy were mild compared with those expected with traditional cytotoxic chemotherapy. Patients in whom CEP-701 showed clinical activity exhibited improved peripheral blood counts with a decrease in peripheral blast counts and an improvement in normal hematopoiesis.
The current clinical study was designed to evaluate the response to single-agent CEP-701 and to relate the activity and toxicities of CEP-701 treatment to the degree of FLT3 inhibition observed in plasma and cell samples. Thus, the study might provide the important proof-of-principle that continuous inhibition of FLT3 in an identifiable subset of AML patients can lead to clinical benefit. This limited phase 1/2 trial focused on 3 doses of CEP-701—40 mg, 60 mg, and 80 mg—all given twice a day by mouth, based on data from earlier phase 1 studies that determined this dosing would provide plasma concentrations in the micromolar range with minimal adverse effects. All 5 patients in whom clinical activity was evident achieved significant, sustained inhibition of FLT3 phosphorylation as measured by the ex vivo bioassay. In 1 patient, we observed fluctuations in the allelic ratio of mutant to wild-type FLT3 that correlated with the clinical response. Three additional patients achieved excellent FLT3 inhibition induced by CEP-701 but failed to show a clinical response to the drug. This lack of response correlated with inherent cellular drug resistance because the 2 patients from whom leukemia cells were available demonstrated clear resistance to CEP-701 in the in vitro cytotoxicity assay (primary blasts were not available from the third patient). Five of the remaining 6 nonresponding patients (2 of whom were treated at a dose level of 40 mg) demonstrated inadequate FLT3 inhibition. In addition to providing possible explanations for the clinical course for most of the patients on this trial, these correlative data raise 2 important issues: appropriate dosing of CEP-701 and inherent drug resistance. CEP-701 is highly protein bound, and our ex vivo bioassay data suggested that submicromolar concentrations of CEP-701 in vivo were generally insufficient to inhibit FLT3 to a level of less than 15% of baseline (despite an IC50 of 2-3 nM in vitro). To achieve an IC90 in vivo, we estimate that plasma concentrations of 5 to 10 μM CEP-701 must be achieved. The data suggest that a dose of 80 mg twice daily may achieve those sustained FLT3-inhibitory plasma drug levels in most patients. Importantly, 80 mg was well tolerated in the 3 patients treated at this dose level, with a therapeutic index that suggests combination with other antileukemic agents is achievable.
Interestingly, 2 of the 8 samples displayed per prima, in vitro resistance to the induction of cytotoxicity by CEP-701, despite more than 90% inhibition of FLT3. Although most primary AML blast samples that harbor FLT3/ITD mutations are selectively susceptible to FLT3 inhibition in vitro, a subset displays a resistant phenotype. To date, we have tested 23 AML primary blast samples harboring FLT3/ITD mutations and found that 19 (83%) were susceptible to FLT3 inhibitors (M.L., unpublished data, October 2002). The etiology of intrinsic resistance remains unclear. However, it appears that some blasts expressing FLT3/ITD mutations have activation of additional pathways that circumvent dependency on FLT3 signaling. In these patients' cells, inhibiting FLT3 alone is not sufficient to induce a cytotoxic response. Thus, FLT3 inhibition alone may be clinically ineffective in some portion (approximately 20%) of patients with AML harboring FLT3 mutations. Inhibiting FLT3 in those patients might prove beneficial when combined with chemotherapy or other therapeutics. Incorporating the cytotoxicity assay into the screening process may help identify patients likely to benefit from the therapeutic approach of using FLT3 inhibitors as monotherapy.
Care should be taken when interpreting the clinical effects of CEP-701 that were generally modest in degree and transient. However, even more thoroughly clinically tested treatments for leukemia, such as all-trans retinoic acid in acute promyelocytic leukemia or imatinib mesylate (Gleevec; Novartis, Basel, Switzerland) in blast crisis chronic myeloid leukemia, are not curative as single agents. Our trial focused on determining whether FLT3 activity could be inhibited in vivo by CEP-701, whether this could be achieved with an acceptable adverse effects profile, and whether antileukemic activity resulted. It seems likely that CEP-701 and other FLT3 inhibitors currently in clinical trials will have to be used in conjunction with established AML chemotherapy regimens or combined with additional signal transduction inhibitors to ultimately improve outcomes in AML with FLT3 mutations. Modeling combination therapy with CEP-701 is ongoing39 and will serve as the basis for combining Ara-C–based chemotherapy with CEP-701. We plan to start with CEP-701 at a dose of 80 mg twice a day and to explore higher doses based on the limited toxicities noted in this trial. As the development of CEP-701 and other FLT3 inhibitors proceeds, monitoring the inhibition of target phosphorylation will be essential for the accurate interpretation of the clinical effects of these compounds. The ex vivo bioassay reported here as a surrogate measure of FLT3 inhibition assumes great importance, particularly in the setting of chemotherapy combination trials, in which peripheral blast numbers will be too low to accurately assay FLT3 phosphorylation directly. Similar surrogate assays may also prove useful in the development of other small molecule enzyme inhibitors, particularly in malignancies in which tumor tissue is difficult to obtain over multiple time points during treatment.
In summary, CEP-701 is a safe, well-tolerated oral compound that can inhibit FLT3 autophosphorylation in vivo to less than 10% to 15% of baseline levels at the doses used in this study. The blasts of most patients with AML harboring FLT3-activating mutations demonstrated in vitro susceptibility to CEP-701. In this subset of patients, successful FLT3 inhibition in vivo correlated with clinical responses. This is the first trial to assess the efficacy of an FLT3 inhibitor in AML, and it is hoped that it represents another step in the development of an important new molecularly targeted therapy for this disease.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-11-3775.
Supported by grants from the National Cancer Institute (CA70970, P50 CA100632, CA91177 [D.S.]; K23 CA81262-01A1 [B.D.S.]; 1K08 CA95600 [M.L.]), the Leukemia and Lymphoma Society (D.S.), and the Sidney Kimmel Foundation (M.L.).
D.S. is the Douglas Kroll Research Foundation Researcher of the Leukemia and Lymphoma Society.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Peter Brown, Michele Jenkins, Lesley Russell, Edward Hellriegel, Craig Dionne, Susan Jones-Bolin, and BruceRuggieri of Cephalon, Inc. for providing financial support of the clinical trial and CEP-701 to the study participants, lending trial management support, and performing the pharmacokinetic analysis. We also thank Drs Judith E. Karp and Robert A. Brodsky for their thoughtful review of the manuscript. Finally, this work would not have been possible without the physicians, nurses, and house staff responsible for the excellent care of the patients.

![Figure 1. Cytotoxicity assay. Bone marrow mononuclear cells from 8 patients treated with CEP-701 were incubated in vitro (in cell culture medium) with increasing concentrations of CEP-701. Each drug concentration point was performed in triplicate. At 72 hours, metabolic activity was determined using the MTT assay. Results are plotted for each sample as percentage (optical density [OD]) of untreated control. Error bars represent SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/10/10.1182_blood-2003-11-3775/6/m_zh80090460740001.jpeg?Expires=1767746407&Signature=xl7id9V32NCDR~l9PlCpXtjO9Vk6BkOibnqeGeGlNrtnERozIY362I-CRG4g~~-W2ejOA7NTNMqhRcIFj-88yOJjNXZ1i3hQATbwysCn9d9HLE0QcJfyzYx8Xtq5Cqr2Tfssfp8pwvAZYFGCq~irpw6awT5qAGEnhEcbgukOY86cIczlf3lBqNnW12sBa8QzlicMYxpUQR5Bcy2L2YwiBsEE8l0UeYt0KYDe3xCM8TEYIrXONHsDSEF1~pzT3RyVxmwRlBv9OcoVRUIeDVYsfGhmn4zrInTBqU~jkkJb3Sl1iBtZi5gCXHSlHLB9dC5GvhYH2--LZcRUiYJ1bFk8uw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Cytotoxicity assay. Bone marrow mononuclear cells from 8 patients treated with CEP-701 were incubated in vitro (in cell culture medium) with increasing concentrations of CEP-701. Each drug concentration point was performed in triplicate. At 72 hours, metabolic activity was determined using the MTT assay. Results are plotted for each sample as percentage (optical density [OD]) of untreated control. Error bars represent SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/10/10.1182_blood-2003-11-3775/6/m_zh80090460740001.jpeg?Expires=1767746408&Signature=AREbkAwj9vERAzpTaahCr8fUBU00B3-x-Bd8ku49Ev8FzQoexAlO35OAvIJSCZMY1Py158SvYl-uRcBMmi0IkBKRikQJwybG4H5aqm73BMHfya4U5qACFVBZh7FHtqaIEZIOasj2nWjyfDIXFclbYjdUdDcxQvzXQnFuE5hZ2s5uXtFTZMA3mmsEqW-x~kuyTKKm7N~w4ibHwGTajV1i-7EQ9BjidkxB1LITrYx-LlOsZJhZRnvM8y5FbL6UM1OCOUMzbHO-YkPuxlNammwbitsiyupLYVas3c8FZV7AvhLEUovjur2UpENTsW9HCsGaQD00qVF7zoOm4luJKD11fg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)