Abstract
Cytotoxic T cells (CTLs) and natural killer cells play a major role in the immune response to Epstein-Barr virus (EBV) infection. In X-linked lymphoproliferative (XLP) disease, a severe immunodeficiency, immunodysregulatory phenomena are observed following EBV infection, suggesting that defects exist in these effector populations. The gene defective in XLP is SAP (signaling lymphocytic activation molecule [SLAM]–associated protein), an adaptor protein that mediates signals through SLAM and other immunoglobulin superfamily receptors including 2B4. We generated EBV-specific T-cell lines from controls and XLP patients and examined CTL function in response to different stimuli. We show that XLP patients can generate EBV–T-cell lines that are phenotypically similar to those from controls. XLP patient EBV–T-cell lines showed a significant decrease in interferon-gamma (IFN-γ) production in response to 2B4 and autologous EBV-transformed lymphoblastoid cell line (LCL) stimulation but not in response to SLAM. Furthermore, XLP EBV–T-cell lines demonstrated markedly decreased cytotoxic activity against autologous LCLs. By retroviral gene transfer of the SAP gene into XLP EBV–T-cell lines, we show reconstitution of IFN-γ production and of cytotoxic activity confirming SAP-dependent defects. These studies demonstrate that in XLP the lack of SAP affects specific signaling pathways resulting in severe disruption of CTL function.
Introduction
X-linked lymphoproliferative disease (XLP), or Duncan disease, is an inherited syndrome characterized by immunedysregulatory phenomena typically following Epstein-Barr virus (EBV) infection that leads to severe infectious mononucleosis, acquired hypogammaglobulinemia, and/or malignant lymphoma.1 The defective gene in XLP has been identified as src homology 2 domain protein 1A (SH2D1A),2 also known as signaling lymphocytic activation molecule (SLAM)–associated protein (SAP) gene.3 SAP is thought to be involved in the coordination of the immune response to EBV or other viral infections. SAP is expressed in natural killer (NK), CD4+, and CD8+ T cells3-5 but not in monocytes6 and primary B cells, although expression in certain B-cell lines has been documented.7
The ligand for SAP was initially defined as SLAM (CD150), a member of the immunoglobulin (Ig) superfamily and a costimulatory molecule found on the surface of T and B lymphocytes and dendritic cells.8 SLAM is a self-ligand and has a number of diverse functions including T/B-lymphocyte costimulation,9 regulation of T-cell cytotoxicity,10 and induction of interferon-γ (IFN-γ) in T helper 1 (Th1) clones.8 The binding of SAP to specific cytoplasmic tyrosine motifs of SLAM serves to block the recruitment of the src homology 2 domain–containing protein tyrosine phosphatase to SLAM and thus may prevent phosphatase activity at the cell membrane. SAP also binds to other Ig superfamily members, including 2B4, which is expressed on NK cells and cytotoxic T cells and is a molecule implicated in the regulation of T- and NK-cell cytotoxicity11,12 ; Ly-9, which is found on mature murine T cells, B cells, and thymocytes; and CD84, which is expressed at low levels on human T cells.13 Recent molecular models of SAP function suggest that at least in murine cells SAP binds to the src homology 3 domain of the phosphotyrosine kinase FynT14-16 and is necessary for recruitment of this molecule to SLAM. However, despite an improved understanding of the structure of SAP and its interactions with these signaling molecules and pathways, the cellular pathogenesis of XLP remains unclear.
In most cases of XLP, EBV plays a critical role as a trigger of pathologic phenotypes. EBV is a human γ-1 herpesvirus that infects most people early in life resulting in infectious mononucleosis (IM), a systemic illness characterized by the proliferation of EBV-infected B lymphocytes and unusually strong NK, CD4+, and CD8+ virus-specific cytotoxic T lymphocyte (CTL) responses. In XLP it has been suggested that the inability of the immune system to control EBV-infected B-lymphocyte proliferation may be due to defects of Th cells, cytotoxic T cells, and/or NK cells. Studies of NK cells from XLP patients show failure of 2B4-mediated cytotoxic killing of CD48-expressing target cells and EBV-immortalized lymphoblastoid B-cell lines (LCLs).12,17,18 This defect is thought to arise from the delivery of an inhibitory signal through the 2B4/CD48 interaction, since disruption of this association restores normal cytolytic function.17
Studies on specific T-cell immunity to EBV in XLP are contradictory. EBV-specific memory T-cell activity as measured by inhibition of autologous LCL outgrowth (regression assay) was defective in the majority of XLP patients studied by Harada et al,19 but other studies claim normal EBV-specific HLA-restricted cytotoxic activity,20 although no specific blocking experiments were undertaken. The production of IFN-γ has been studied by a number of groups but is again inconclusive. Although Yasuda et al21 demonstrated that T cells from XLP patients exhibit a significant decrease in IFN-γ production, in another study mononuclear cells from 1 XLP patient during acute EBV infection showed elevated levels of serum IFN-γ compared with EBV-seropositive healthy individuals.22,23 More recently it was shown that Herpesvirus saimiri–transformed CD4+ T cells from XLP patients exhibited severe defects in up-regulation of IFN-γ and interleukin-2 (IL-2) on T-cell receptor and SLAM stimulation and mixed lymphocyte reactions in comparison with controls.24 In murine SAP “knockout” models, lymphocyte development is normal, but challenge with lymphocytic choriomeningitis virus (LCMV) or Toxoplasma results in increased T-cell activation, proliferation, and excessive IFN-γ production with a bias to a Th1 T-cell profile.5,25 Together, these human and murine studies suggest that disturbance of the Th1/Th2 cytokine balance and dysregulation of T-lymphocyte cytotoxic function may be crucial to the cellular pathogenesis of the condition, but as yet no data have been reported in a clinically relevant model.
To understand the cellular defects in XLP we developed an autologous EBV-LCL/EBV–T-cell line model from XLP patients and studied the phenotypic profile and functionality of effector T cells. We show that in response to specific stimuli, the IFN-γ response in XLP patients is defective and that, although T cells from XLP patients are capable of generating EBV-specific T-cell lines (EBV–T-cell lines), they lack cytotoxic activity against autologous LCLs. Finally, using a retrovirus gene delivery system encoding human SAP cDNA, we demonstrate reconstitution of cytokine secretion and cytotoxic function in patient EBV–T-cell lines, thereby confirming SAP-dependent cellular defects in XLP.
Materials and methods
Generation of SAP-expressing retroviral vector
Human SAP(SH2D1A) cDNA (Online Mendelian Inheritance in Man [OMIM] no., AL023657) cloned into pGEM-T (Promega, Madison, WI) was a gift from Dr Alison Coffey (The Sanger Center, Cambridge, United Kingdom). SAP cDNA was amplified using the following primers. The forward primer, SAP-F, containing a SalI restriction site (underlined), was placed upstream of the initiation codon and the reverse primer, SAP-R, downstream at codon 553 but upstream of the polyA+ signal. The primers used are as follows: SAP-F, GCC CAA GAGTCGACC AGG CCA TGG; SAP-R, GTA CAA GGT GTT TTA GTC GAC TTC ATG GGG GCT TTC.
The 400–base pair (bp) product was digested with SalI and cloned into plasmid Blue script (pBS; Promega), sequenced, and then subcloned into the retroviral backbone. A murine replication-deficient oncoretroviral vector containing the U3 modified 3′ long terminal repeat (LTR) from spleen focus-forming virus (SFFV), 5′LTR from PCMV (PCC4 cell passaged murine sarcoma virus),26 and primer binding site (pbs)27 was used for enhanced hematopoietic cell tropism and reduced transgene silencing. Human SAP cDNA was cloned upstream of the encephalomyocarditis virus–derived internal ribosomal entry sequence (IRES) sequence and red-shifted variant of the enhanced green fluorescent protein (eGFP). Vectors containing only eGFP were generated for controls in all experiments. The PG13 packaging cell line (providing the Gibbon ape leukemia virus envelope protein) was used to generate replication-defective virus. One clone producing virus at 107 transducing units/mL was selected.
Purification of PBMCs from healthy and XLP patients
This study was approved by the institutional research ethics committee and informed consent was obtained from all subjects before taking blood. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density centrifugation from anticoagulated whole blood derived from 3 healthy donors (C) and 3 XLP patients (P1, P2, and P3). The 3 patients were all from different kindreds and each had a different mutation in the SAP gene, which led to the absence of SAP expression.6 The clinical phenotype of each patient was different: P1 was EBV seropositive and previous fulminant IM, P2 was EBV seropositive and previous B-cell lymphoma treated with chemotherapy, and P3 was EBV seronegative and had dysgammaglobulinemia.
Generation and culture of EBV–T-cell lines
EBV-transformed LCLs were generated from healthy donors and EBV-seropositive XLP patients (P1 and P2) using standard techniques. EBV–T-cell lines were generated by stimulating 1 × 106/mL PBMCs cells with 2.5 × 104/mL autologous LCLs (40:1 cell ratio) using standard culture conditions.28 Cells were washed, recultured at a concentration of 1 × 106/mL, and restimulated with 2 × 105/mL autologous LCLs at day 9. Cells were either prepared for gene transfer on day 10 or kept in culture with restimulations weekly at an effector-target ratio of 4:1. A total of 20 IU IL-2 (Proleukin; Chiron, Emeryville, CA) was added to the cultures for the first time at day 10 and twice weekly thereafter. All cell lines were cultured up to 8 to 10 weeks. EBV–T-cell lines with mainly a CD4+ phenotype were produced by stimulation with phytohemagglutinin (PHA, 5 ng/mL) and IL-2 (20 IU/mL) at day 7 and continued in the same conditions as described above for generation of CD8+ EBV–T-cell lines. The data for enzyme-linked immunospot (ELISPOT) and cytotoxicity experiments are from studies on CD8+ EBV–T-cell lines.
Gene transfer
EBV–T-cell lines (days 9-10) were placed in retronectin-coated wells loaded with virus supernatant. Retronectin (Takara Biomedicals, Shiga, Japan) at 18.5 μg/mL was coated on non–tissue culture–treated plates for 2 hours at 37° C in 24-well plates. Plates were blocked with 1% human serum albumin for 60 minutes and washed twice with PBS. Cells were plated at a concentration of 1 × 106/mL for 24 hours. Half of the supernatant was replaced with fresh medium containing 10% heat-inactivated fetal calf serum (FCS) and 20 IU/mL IL-2. A second round of virus exposure was performed after 24 hours in the same conditions. Cells were maintained in culture at a concentration of 1 × 106/mL to 1.5 × 106/mL. Cells were analyzed 3 days later by flow cytometry for expression of the reporter gene eGFP. Cell lines were sorted after 4 weeks of stimulation to reach maximum transduced cell purity.
LDH release cytotoxic assay
Cytotoxicity of EBV–T-cell lines was measured using lactate dehydrogenase (LDH) release assays (Promega). Briefly, autologous LCLs were used as target cells with EBV–T-cell lines as effector cells at different effector-target cell ratios. All targets were plated in triplicate. After a 4-hour incubation, supernatants were harvested and the specific cytotoxicity was determined using a microplate enzyme-linked immunosorbent assay reader (Dynatech Labs, Chantilly, VA). The percentage of specific lysis was calculated as 100% × (experimental release–spontaneous release)/(maximum release–spontaneous release). Maximum release was obtained by adding 100 μL of 5% Triton X-100 to the 100 μL medium containing target cells. Spontaneous release was consistently less than 15% of maximum release in all assays.
ELISPOT assay
The ELISPOT assay was performed as described29 with some modifications. PBMCs and EBV–T-cell lines were plated at 1 × 104/well. Responder cell populations were seeded across a range of concentrations to achieve 10 to 100 spots/well so as to facilitate accurate and reproducible counting. For LCL stimulators with PBMC responders, the concentration used was 5 × 103 LCLs to 2 × 104 PBMC/well; for stimulators with EBV–T-cell line responders, this was 5 × 103 LCLs to 2 × 104 EBV–T-cell lines/well. For antibody stimulation of PBMCs and EBV–T-cell lines, all antibody concentrations used in the ELISPOT assay were optimized at 5 μg/mL: CD3 (OKT3; Janssen-Cilag, High Wycombe, United Kingdom), CD28 (BD Pharmingen, San Diego, CA), α-SLAM (A12 clone; BD Pharmingen), 2B4 (C1.7 clone; Beckman Coulter, High Wycombe, United Kingdom), and as a positive control PBMCs or EBV–T-cell lines stimulated with 2.5 μg/mL phorbol myristate acetate (PMA; Sigma, St Louis, MO) and 1 μg/mL ionomycin (Sigma). All cells were cultured in RPMI-FCS 10% supplemented to a final volume of 200 μL/well. After undisturbed incubation for 24 to 48 hours at 37° C, with 5% CO2, plates were washed 4 times with PBS containing 0.05% Tween 20 (PBS/0.05% Tw). Wells were incubated with biotinylated “detection” antibody against IFN-γ (Mabtech AB, Nacka, Sweden). The plates were washed 6 times with PBS/0.05% Tw. Avidin-peroxidase-complex (AEC, 100 μL; prepared according to manufacturer's instructions; Vector Laboratories, Burlingame, CA) was added per well for 1 hour at room temperature. The plates were washed 3 times with PBS/0.05% Tw, followed by 3 washes with PBS. AEC substrate (Sigma) was prepared according to the manufacturer's instructions and filtered through a 0.45-μM filter prior to use. Per well, 100 μL was added. After 4 minutes the reaction was stopped with deionized water and the plates were dried overnight prior to membrane removal. The spot number was determined in an independent blinded fashion (Bioreader 3000; Bio-Sys, Karben, Germany). In each experiment, the result was expressed as spots/1000 cells and the results of 3 experiments were used to calculate means and standard deviations.
Statistical analysis
Control values for each experiment (Figures 2,4) were derived from pooled data on a number of control individuals so that each experiment has a single control value, mean, and standard deviation. For experiments in Figure 2, 3 different control individuals were studied each in triplicate, and therefore the control mean is obtained from 9 data points. For experiments in Figure 4, 2 different control individuals were studied each in triplicate, and therefore the control mean is obtained from 6 data points. Each mean patient response and variance was then compared against the control response for each experiment and analyzed for statistical significance using a one-tail unpaired t test, assuming unequal variance between the 2 study groups. Significant differences are indicated by asterisks (no asterisk, no statistical significance; *P < .05; **P < .01; ***P < .001).
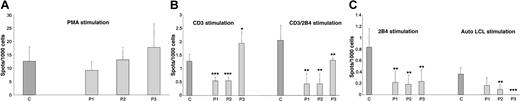
IFN-γ expression by PBMCs from controls and XLP patients following stimulation. ELISPOT analysis was used to determine IFN-γ expression following stimulation of PBMCs from controls (C) and XLP patients (P1, P2, and P3) with (panel A) PMA, (panel B) CD3 or CD3/2B4, and (panel C) 2B4 alone or autologous LCL stimulation. Error bars indicate standard deviation.
IFN-γ expression by PBMCs from controls and XLP patients following stimulation. ELISPOT analysis was used to determine IFN-γ expression following stimulation of PBMCs from controls (C) and XLP patients (P1, P2, and P3) with (panel A) PMA, (panel B) CD3 or CD3/2B4, and (panel C) 2B4 alone or autologous LCL stimulation. Error bars indicate standard deviation.
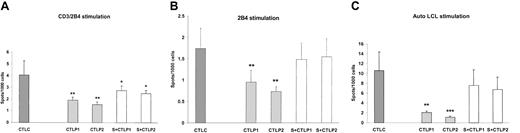
IFN-γ expression by EBV–T-cell lines from controls and XLP patients following stimulation. ELISPOT analysis was used to determine IFN-γ expression following stimulation of EBV–T-cell lines from controls (CTLC) and XLP patients (CTLP1 and CTLP2) and SAP-transduced XLP patient EBV–T-cell lines (S + CTLP1, S + CTLP2) with (A) CD3/2B4, (B) 2B4 alone, or (C) autologous LCL. Error bars indicate standard deviation.
IFN-γ expression by EBV–T-cell lines from controls and XLP patients following stimulation. ELISPOT analysis was used to determine IFN-γ expression following stimulation of EBV–T-cell lines from controls (CTLC) and XLP patients (CTLP1 and CTLP2) and SAP-transduced XLP patient EBV–T-cell lines (S + CTLP1, S + CTLP2) with (A) CD3/2B4, (B) 2B4 alone, or (C) autologous LCL. Error bars indicate standard deviation.
SAP immunoblotting
Cell lysates were prepared from SAP-reconstituted and nonreconstituted EBV–T-cell lines from patients and healthy donors, according to previous protocols. The lysates (1 × 106 cells) were fractionated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and analyzed by blotting onto nitrocellulose (MSI, Bedford, MA), blocking with 2.5% milk in PBS/0.1% Tw, and immunoblotting with anti-SAP antibody generated against the C-terminal end of SAP protein7 at a final concentration of 1:1000. The membrane was washed with PBS/0.1% Tw and incubated with a horseradish peroxidase–conjugated antirabbit antibody (Sigma) and washed again before enhanced chemiluminescence (ECL; Amersham Biosciences, Amersham, United Kingdom) detection.
Flow cytometric analysis and FACS
Flow cytometry of EBV–T-cell lines was performed using an EPICS XL flow cytometer (Beckman Coulter) and antibodies for CD3, CD4, CD8, CD16, CD25, CD27, CD28, CD45RA, CD45RO, CD56, CD69, CD95 (Fas), CDw150 (SLAM), CD244 (2B4), T-cell receptor αβ (TCRαβ), and TCRγδ (all from BD Pharmingen).
Gene-modified EBV–T-cell lines were purified using an EPICS Altra fluorescence activated cell sorter (FACS) (Beckman Coulter). Cells were stained with phycoerythrin (PE)–conjugated CD3 (BD Pharmingen) and analyzed for PE-CD3 and eGFP expression. Dual-color positive cells (positive fraction) and single-color CD3 cells (negative fraction) were sorted to high purity (more than 99%). One unsorted fraction was retained. The 3 cell fractions were subsequently used separately for cytotoxicity assays.
MHC class I blocking of target cells
W6/32 monoclonal antibody (mAb; a gift from Dr Bin Gao, ICH, London, United Kingdom) was used to block major histocompatibility complex (MHC) class I antigen presentation on LCLs. Cells were washed twice with PBS and then incubated with W6/32 mAb (1 μg/mL) on ice for one hour. The cells were then washed and used in cytotoxicity assays.
Results
Immunophenotypic characteristics of EBV–T-cell lines from XLP patients
The cellular response to EBV infection is mediated predominantly by CD8+ cytotoxic T cells, which retain memory and provide lifelong immunity against EBV. The detailed immunophenotype and function of EBV–T-cell lines derived from XLP patients has not previously been reported. Using autologous LCLs as stimulators, EBV–T-cell lines from 2 healthy individuals (CTLC1 and CTLC2) and 2 XLP patients (CTLP1 and CTLP2) were generated. Detailed cell surface analysis of CD8+ and CD4+ EBV–T-cell lines generated from both XLP patients and healthy individuals revealed similar surface marker expression profiles (Table 1). Expression of memory/naive (CD45RO/CD45RA) and activation (CD25 and CD69) markers was similar in cell lines derived from healthy and XLP individuals with comparable mean fluorescence intensity (MFI) for the majority of markers studied.
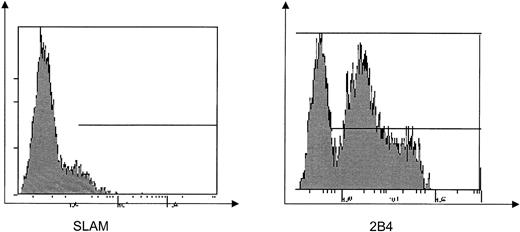
Previous reports have suggested that SLAM is the dominant molecule mediating cytotoxicity in T lymphocytes. However, in this analysis we found very low surface expression of SLAM on EBV–T-cell lines from both healthy and XLP individuals, while in contrast 2B4 is highly expressed on these cell lines (Figure 1; Table 1), suggesting that 2B4 rather than SLAM may be more important in mediating cytotoxicity in EBV–T-cell lines.
Cell surface expression of SLAM and 2B4 on EBV–T-cell lines. Flow cytometric analysis of SLAM and 2B4 expression on control EBV–T-cell lines. Horizontal line indicates expression above background.
Cell surface expression of SLAM and 2B4 on EBV–T-cell lines. Flow cytometric analysis of SLAM and 2B4 expression on control EBV–T-cell lines. Horizontal line indicates expression above background.
IFN-γ production in PBMCs from XLP patients is intact but decreased in response to specific stimuli
IFN-γ production in cells from XLP patients in comparison with healthy individuals was studied in response to a variety of different stimuli. In initial experiments, responses in PBMCs from 3 XLP (P1, P2, and P3) individuals (with defined genetic lesions; “Materials and methods”) were analyzed by ELISPOT assays and compared with responses seen in control samples (C). Following PMA stimulation, cells from XLP patients showed high levels of IFN-γ production with profiles similar to that observed in healthy individuals, suggesting an intact capacity to produce IFN-γ (Figure 2A). Cells were then activated with more specific stimuli. In these specific receptor stimulation experiments we observed significant differences in IFN-γ production between controls and patient responses. After stimulation with CD3, CD3/2B4, and 2B4, significantly less IFN-γ secretion was observed in patient samples compared with controls (Figure 2B-C). One exception to this was P3 (who was EBV seronegative at the time of analysis), who showed an increased IFN-γ response following CD3 stimulation. His responses to CD3/2B4 and 2B4 were similar to the other patients. Cells were then stimulated with autologous EBV-immortalized LCLs and again the IFN-γ was decreased in all patients, except in P3 who was EBV seronegative and where no spots were observed—this would be expected due to lack of memory to EBV (Figure 2C). Cells were also stimulated with CD28, CD3/CD28, SLAM, or CD3/SLAM, but no differences were observed between control individuals and XLP patients (data not shown).
EBV–T-cell line function in XLP and reconstitution of defects following SAP gene transfer
It has been postulated that the cellular pathogenesis of XLP may reside in abnormal function of EBV–T-cell lines and in their inability to control EBV infection. Previous reports, performed before the identification of the SAP gene defect, are inconclusive with certain studies demonstrating that cytokine secretion and cytotoxicity are abnormal and others suggesting that both functions are intact. We examined whether introducing expression of the SAP gene into EBV–T-cell lines from XLP patients (known to lack SAP expression) could restore these observed functional defects. We transduced EBV–T-cell lines from XLP patients P1 and P2 (CTLP1 and CTLP2) with a retroviral vector encoding the SAP cDNA and the reporter eGFP (SAP-transduced cells designated S + CTLP1 and S + CTLP2). Transduced EBV–T-cell lines were analyzed and showed the same phenotypic characteristics as the parent cell lines (data not shown). The expression of eGFP and SAP in the transduced cell lines was confirmed by flow cytometric and immunoblot analysis, respectively (Figure 3A-B). The percentage of cells transduced was approximately 45%, and these cells were purified by FACS for use in the reconstitution experiments described in Figures 4 and 5.
eGFP and SAP expression following retroviral transduction of EBV–T-cell lines from XLP patients. (A) Flow cytometric analysis of EBV–T-cell lines from XLP patient P1 following transduction with eGFP/SAP-encoding retroviral construct shows 42% expression of eGFP in the EBV–T-cell line from patient 1 (S + CTLP1). (B) Immunoblot analysis using an anti-SAP antibody shows a 15-kDa protein expressed in EBV–T-cell lines from a control (CTLC1) and in these cells transduced using the eGFP/SAP retroviral vector (S + CTLC1) and a vector encoding eGFP only (G + CTLC1). The 15-kDa protein is also seen in XLP EBV–T-cell lines transduced with the eGFP/SAP retroviral vector (S + CTLP1) but not in EBV–T-cell lines transduced with the vector encoding eGFP only (G + CTLP1) or in untransduced EBV–T-cell lines (CTLP1).
eGFP and SAP expression following retroviral transduction of EBV–T-cell lines from XLP patients. (A) Flow cytometric analysis of EBV–T-cell lines from XLP patient P1 following transduction with eGFP/SAP-encoding retroviral construct shows 42% expression of eGFP in the EBV–T-cell line from patient 1 (S + CTLP1). (B) Immunoblot analysis using an anti-SAP antibody shows a 15-kDa protein expressed in EBV–T-cell lines from a control (CTLC1) and in these cells transduced using the eGFP/SAP retroviral vector (S + CTLC1) and a vector encoding eGFP only (G + CTLC1). The 15-kDa protein is also seen in XLP EBV–T-cell lines transduced with the eGFP/SAP retroviral vector (S + CTLP1) but not in EBV–T-cell lines transduced with the vector encoding eGFP only (G + CTLP1) or in untransduced EBV–T-cell lines (CTLP1).
EBV–T-cell line cytotoxicity against autologous LCL in controls and XLP patients. Cytotoxicity of untransduced and transduced EBV–T-cell lines from controls and XLP patients was measured using LDH release assays. (A) EBV–T-cell lines from C1 untransduced (CTLC1), eGFP/SAP-vector transduced (S + CTLC1), and eGFP alone vector (G + CTLC1); (B) the same as for panel A, except using CTLC2; (C) the same as for panel A, except using CTLP1; (D) the same as for panel A, except using CTLP2. (E) Incubation of eGFP/SAP vector–transduced EBV–T-cell lines from both controls and XLP patients (S + CTLC1, S + CTLC2, S + CTLP1, S + CTLP2) with W6/32 antibody.
EBV–T-cell line cytotoxicity against autologous LCL in controls and XLP patients. Cytotoxicity of untransduced and transduced EBV–T-cell lines from controls and XLP patients was measured using LDH release assays. (A) EBV–T-cell lines from C1 untransduced (CTLC1), eGFP/SAP-vector transduced (S + CTLC1), and eGFP alone vector (G + CTLC1); (B) the same as for panel A, except using CTLC2; (C) the same as for panel A, except using CTLP1; (D) the same as for panel A, except using CTLP2. (E) Incubation of eGFP/SAP vector–transduced EBV–T-cell lines from both controls and XLP patients (S + CTLC1, S + CTLC2, S + CTLP1, S + CTLP2) with W6/32 antibody.
CD8+ EBV–T-cell lines from healthy individuals (C), XLP patients untransduced (CTLP1, CTLP2), and SAP transduced (S + CTLP1, S + CTLP2) were then activated with the same set of stimuli used in the PBMC experiments described in Figure 2. Following PMA, CD3, CD28, CD3/CD28, SLAM, and CD3/ SLAM stimulation no difference in IFN-γ secretion was observed between control EBV–T-cell lines and untransduced and transduced XLP EBV–T-cell lines (data not shown). Cells were then stimulated with CD3/2B4, 2B4, or autologous LCLs using a range of effector-target cell ratios. Figure 4A-C shows that in comparison with the control CTL response, there is significantly decreased IFN-γ secretion observed in XLP EBV–T-cell lines from both patients following all 3 stimuli. However, SAP reconstituted lines, S + CTLP1 and S + CTLP2, show marked increases in IFN-γ secretion that, following 2B4 or autologous LCL stimulation, are not statistically different from the control EBV–T-cell line response. The most dramatic reduction in IFN-γ secretion and reconstitution of function is seen following autologous LCL stimulation (Figure 4C). As a further control, XLP EBV–T-cell lines transduced with eGFP alone did not show any differences in IFN-γ secretion, suggesting that the process of retroviral transduction per se did not affect cytokine secretion (data not shown).
We next tested the ability of XLP EBV–T-cell lines to kill autologous LCLs using a standard LDH release assay (Figure 5). EBV–T-cell lines from both XLP patient P1 and P2 showed markedly decreased cytotoxic activity in comparison with EBV–T-cell lines from 2 healthy individuals (Figure 5A-D). SAP reconstitution experiments were then performed to see if cytotoxic function could be restored to XLP EBV–T-cell lines. We demonstrate that cytotoxic function can be increased from 30% to 60% cell lysis for CTLP1 (Figure 5C), and from 20% to 60% for CTLP2 (Figure 5D), at effector-target cell ratios of 10:1. At similar effector-target cell ratios, approximately 80% cell lysis was seen in EBV–T-cell lines from healthy individuals C1 and C2 (Figure 5A-B). Normal EBV–T-cell lines transduced with SAP or eGFP (S + CTL or G + CTL) or XLP EBV–T-cell lines transduced with eGFP (G + CTLP) did not show any differences in cytotoxic activity from untransduced cell lines, again suggesting that retroviral transduction per se did not affect cytotoxic activity. To confirm MHC class I–specific mediated killing, incubation with an MHC class I antibody reduced cytotoxic activity to 20% to 30% cell lysis in both normal and S + CTLP lines (Figure 5E).
Discussion
Following the identification of SAP as the defective gene in XLP, there has been considerable progress in the understanding of the SAP protein structure, its interaction with cell surface molecules and more recently with proximal tyrosine kinases such as FynT.15,16 However, the cellular pathogenesis of XLP in humans remains poorly understood, especially given the different clinical phenotypes of the disease. Murine models of SAP deficiency suggest a tendency to dysregulated Th1 responses with T-cell activation and increased IFN-γ production, but it is clear from studies of other immunodeficiencies that abnormalities observed in murine models do not always accurately reflect human disease.30 It is evident from a number of reports that human NK cell defects are present in this condition,17,18,31 but there are very little clear functional data on specific T-cell populations. In this study the use of EBV–T-cell lines from XLP patients represents a physiologic human T-cell effector population. The lack of cellular transformation or species differences provides a relevant model for examining defects in this condition.
In healthy individuals the immune response to EBV infection, the major pathologic trigger in XLP, is dominated by the proliferation of CD8+ EBV–T-cell lines and NK cells. The development of EBV-driven lymphoma and fulminant IM in XLP32 would strongly suggest that defects exist in these effector populations. In this study we show that XLP patients are indeed capable of generating EBV–T-cell lines. Using different culture conditions we were able to generate T-cell lines of both CD8+ and CD4+ phenotype from both healthy and XLP patients. Although most cytotoxic T-cell lines are CD8+, polyclonal expansion of T cells in response to viral antigens33 can result in CD4+ T-cell lines and CD4+ cytotoxicity is well documented.34 Cell surface marker expression profiles and intensity of expression were similar in both XLP and control groups. In both controls and XLP EBV–T-cell lines there was very little SLAM expression observed, while 2B4 was expressed at significant levels. To date, SLAM has been cited as the main partner for SAP binding and as an important costimulatory molecule in T-cell responses to antigenic stimulation. Much of this work has been performed on murine T cells and the differential expression of SLAM and 2B4 in human effector cells has not been extensively studied. Our analysis shows abundant 2B4 expression in both control and XLP EBV–T-cell lines, while SLAM expression was minimal. Furthermore, in preliminary studies, stimulation of EBV–T-cell lines and PBMCs from healthy individuals shows no significant enhancement in SLAM expression (R.S., H.B.G., unpublished data, 2004). Our observations would suggest that 2B4, not SLAM, is the major partner for SAP in EBV–T-cell lines and, as previously observed in NK cells, signaling through 2B4 in XLP EBV–T-cell lines may be defective.
A number of studies have reported cytokine abnormalities in XLP. However, in the majority of studies undertaken, the response to specific cell surface stimuli has not been addressed. In this analysis we stimulated both PBMCs and XLP EBV–T-cell lines with specific T-cell stimuli and assessed the IFN-γ response. In both populations, differences were observed in response to CD3, CD3/2B4, 2B4, or autologous LCLs and not in response to PMA, CD28, CD3/CD28, SLAM, or CD3/SLAM. The lack of response to CD3/SLAM or SLAM alone would be in keeping with the low levels of SLAM observed in these cell lines.
Following CD3 stimulation alone, P1 and P2, who are EBV experienced, demonstrate significant down-regulation of IFN-γ production. This is in keeping with observations made by Sanzone et al where EBV-experienced XLP patients were analyzed.35 However, P3 (who was EBV seronegative at the time of analysis) shows an exaggerated IFN-γ response that is significantly elevated from controls and is similar to the CD3 stimulation T-cell responses observed in SAP-deficient mice.5,25 Importantly stimulation via 2B4 or CD3/2B4 in P3 shows a down-regulated response and is similar to P1 and P2. These observations, albeit in only one patient, suggest that SAP deficiency in the EBV-inexperienced individual augments CD3 stimulation, which after EBV infection is down-regulated. The role of SAP downstream of CD3 triggering without costimulatory input is unclear, but these data suggest a regulatory role for SAP that needs further investigation in larger numbers of patients.
In our study, differences in XLP and normal EBV–T-cell lines following CD3/2B4 or 2B4 activation suggest that activation through this specific pathway is important in EBV–T-cell line function and, given the similarities in the cell surface phenotype between the 2 T-cell populations, point to defective SAP expression as being responsible for aberrant transduction of the 2B4 signal. Similar findings after stimulation by autologous LCLs are consistent with these findings. Cell surface LCL/EBV–T-cell line interactions include homotypic association between SLAM molecules (although in this study low SLAM expression negates this pathway) and also between 2B4 on EBV–T-cell lines with its ligand CD48, which is abundantly expressed on LCLs. Disruption of the signal arising from this interaction results from the lack of SAP expression in XLP EBV–T-cell lines probably leading to decreased IFN-γ expression and to the observed failure of cytotoxicity of autologous LCLs.
Our studies on human cells from EBV-experienced individuals demonstrate a decrease in IFN-γ production in response to specific stimuli and contrast significantly with murine data. A possible explanation for this discrepancy may be the fact that in murine studies, animals were examined at an early age and only after limited antigenic exposure. It would be interesting to repeat these studies on mice exposed to repeated or chronic antigen stimuli. This would be a better representation of the situation in XLP, which was initially described as a “progressive” immunodeficiency and where repeated immunologic insults and more specifically with EBV may result in a gradual deterioration in immune function.
We demonstrate that human EBV–T-cell lines from 2 patients with a molecular diagnosis of XLP and lack of SAP expression show significant defects in direct killing of autologous LCLs. Previous studies have suggested this using indirect assays such as LCL growth regression. That this defect occurs via the 2B4/CD48 pathway is supported by the abundance of 2B4 rather than SLAM expression and also by the demonstration of 2B4/CD48 abnormalities in NK cell cytotoxicity in XLP.18 The molecular detail of this pathway is unclear, but it has been shown that 2B4 associates with the LAT (linker for activation of T cells) leading to tyrosine phosphorylation of both molecules. Whether this interaction is SAP dependent has yet to be defined. It is also evident that other receptors are involved in mediating cytotoxicity in T cells. NTB-A is a recently identified member of the SLAM family and can bind SAP following phosphorylation of its cytoplasmic tail.36 NTB-A behaves in an analogous manner to 2B4 in NK cells triggering cytotoxicity but is also expressed in T cells suggesting that the observed defects in XLP EBV–T-cell lines may arise from abnormal signaling downstream of a number of different surface receptors. The final events in these pathways may be the inability to form the cytolytic machinery such as release of granzyme or perforin molecules, and expression of such proteins in XLP EBV–T-cell lines is currently being investigated.
The cytokine expression and cytotoxicity defects are SAP dependent since introduction of SAP by gene transfer restored functionality. A marked increase was seen in both patients for cytotoxicity and cytokine assays. The restoration of function was equivalent to control samples and mock-transfected cells did not show any reconstitution.
Retroviral transfer of the SAP gene into EBV–T-cell lines was achieved during the repeated stimulation of T cells by autologous LCLs and makes CTLs an attractive target for gene transfer. Although the studies were principally conducted to show that the defects were SAP dependent, the restoration of function suggests that SAP gene transfer in XLP may hold some therapeutic value. In cases without a matched sibling donor, gene-corrected CTLs could be generated and stored for future use if lymphoma or fulminant IM were to arise in XLP patients. However, much further work would be necessary to show that constitutive SAP expression under the control of a viral LTR does not lead to adverse effects. The possibility of SAP expression under the control of a T-lineage–restricted or endogenous SAP promoter could also be explored.
To date, functional cytotoxic T-cell defects in XLP have not been carefully defined, especially in response to EBV infection. Using this autologous EBV-LCL/EBV–T-cell line model we clearly demonstrate that both cytokine production and cytolytic activity are severely impaired as a result of SAP deficiency. In combination with previously described NK cell defects this may explain the failure to control EBV infection and predisposition to B-cell lymphoma, which occurs in one third of XLP patients and that indeed was the presenting feature of XLP patient P2. One may also speculate that the tendency to fulminant IM (as in P1) and hemophagocytosis may possibly arise from the initial CTL and NK cell failure leading to further dysregulated immune responses.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-09-3359.
Supported by the Primary Immunodeficiency Association, and the University College London (R.S.). A.J.T. is supported by the Wellcome Trust.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This manuscript is dedicated to the memory of Jonathon May and his family for their invaluable help in the undertaking of this study. We are grateful to Ross Gilmour for help with statistical analysis.