Abstract
Recovery of dendritic cells (DCs) and natural killer (NK) cells after allogeneic stem cell transplantation (SCT) is important for allograft responses and antitumor immunity and thus for treatment outcome. Regulation of this regenerative process is not well understood. We investigated the influence of endogenous cytokines on the recovery and diversification of DC and NK cell subsets up to 6 months after SCT. Reconstitution of circulating DCs and NK cells was rapid but accompanied by prolonged skewing of cell subsets. The speed of recovery of CD11c+CD123low DC1 exceeded that of CD11c– CD123+ DC2, and correlated with plasma levels of flt3 ligand (FL), but not with granulocyte or granulocyte-macrophage colony-stimulating factors and stem cell factor. There was a 5-fold increase in interferon-γ–producing CD56highCD16–/low NK cells and a corresponding reduction in the CD56lowCD16high subset, accompanied by strongly reduced NK cell cytotoxicity. In vitro data implicate an inhibitory effect of cyclosporin A on NK cell differentiation and cytotoxicity. NK cell numbers did not correlate with plasma levels of FL or interleukin 15. Our results demonstrate that endogenous FL has distinct effects on the kinetics of reconstitution of DCs and NK cells and have potential implications for the modulation of immune responses after allogeneic SCT.
Introduction
Early after stem cell transplantation (SCT), immune function is determined by both mature immunocompetent cells transferred with the allogeneic graft and by immune populations that arise de novo from transplanted stem cells. Distinct cell lineages of the developing immune system reconstitute at very different rates. T-cell recovery is slow, determined primarily by thymic function, which declines with age and is additionally damaged by conditioning regimens.1,2 Newly developing dendritic cells (DCs) and natural killer (NK) cells integrate within the first 2 to 3 weeks into the patient's immune system by rapid maturation of engrafted stem cells.3-5 DCs represent a rare cell population important for the initiation and regulation of T cell–dependent immune responses,6 and immediate DC precursors in blood constitute less than 1% of mononuclear cells. NK cells, comprising about 10% of circulating lymphocytes, are important effectors of innate immunity because of their ability to lyse target cells without the need for prior antigen stimulation.7 Both DCs and NK cells represent heterogeneous populations composed of cell subsets with distinct phenotypic and functional characteristics. Human blood DCs belong to CD11c+CD123low or CD11c–CD123+ subpopulations designated accordingly DC1 and DC2,8 reflecting different capacities to stimulate and polarize T-helper (TH) cell responses toward either TH1 or TH2 type, respectively.9 The CD56+CD3– NK cells are composed of a predominant highly cytotoxic CD56dimCD16high subset and a minor population of CD56highCD16–/low cells with low cytotoxicity but abundant cytokine production.10 The functional heterogeneity of DCs and NK cells is likely to affect immune responses after SCT important for stem cell engraftment, tolerance to alloantigens, as well as recognition and eradication of malignant clones. However, understanding of the microenvironmental factors that influence the rapid development and diversification of the subsets of DC and NK cells after allogeneic SCT is very limited.
Flt3 ligand (FL) is a hematopoietic growth factor binding to the flt3 receptor, expression of which is restricted to primitive hematopoietic progenitors.11,12 Mice rendered FL deficient have markedly reduced numbers of circulating and organ-resident DCs and NK cells.13 In vitro, FL supports early steps in the differentiation of human NK cells and DC1 and DC2 from CD34+ progenitors.14-16 When administered in vivo, FL has the unique ability to expand DCs in multiple tissues of the mouse17 and markedly elevates the number of circulating DCs in healthy human volunteers.18 In contrast to treatment with granulocyte colony–stimulating factor (G-CSF), which mobilizes exclusively the DC2 subset,19 or granulocyte-macrophage colony stimulating factor (GM-CSF), which expands selectively myeloid-type CD11c+CD11b+DCs in mice,20 FL administration led to the expansion of both DC1 and DC2 subsets in mice and humans.18,20,21 We have previously shown that engraftment of stem cells after SCT is preceded by a transient but strong increase in serum levels of FL, reflecting a compensatory growth factor response to stem cell deficiency.22,23 This increase is partially blocked in the presence of cyclosporin A (CsA) administered to prevent allograft responses after SCT.24 Given the importance of FL for the development of hematopoietic precursors into functionally mature DCs and NK cells, we hypothesized that up-regulation of the endogenous levels of this cytokine might influence the reconstitution of DC and NK-cell lineages after allogeneic SCT.
In this study, we examined the DC and NK-cell compartments within the initial 6 months after allogeneic SCT. Results demonstrate that rapid reconstitution of circulating DCs and NK cells was accompanied by phenotypic and functional abnormalities of cell subsets. FL had unique influence on the recovery of DC but not NK-cell lineages, which may be of importance for graft acceptance and antileukemic responses after allogeneic SCT.
Patients, materials, and methods
Patients and controls
Twenty-eight patients who underwent an allogeneic SCT were enrolled in our study (Table 1). High-intensity conditioning consisted of cyclophosphamide at 60 mg/kg, followed by total body irradiation at 6 × 2 Gy, preceded in high-risk patients by additional etoposide at 30 mg/kg. Prevention of graft-versus-host disease (GvHD) in all patients was based on CsA (150-300 mg/d) and a short course of methotrexate. Six patients were part of a double-transplantation program.25 Median time to reach the neutrophil count of more than 500/μL was 14 days. On day 30, clinically evident acute GvHD grade I was observed in 8 patients and grade II or III in 14 patients. On day +180, 19 patients were in complete remission, including 1 patient who had rejected the graft and achieved autologous reconstitution. Four patients died of a GvHD, 3 of infections, 1 of graft rejection, and 1 of leukemia relapse. All clinical samples of peripheral blood (PB) and bone marrow (BM) were obtained with informed consent in compliance with the guidelines of the Ethical Committee of the University Hospitals of Basel (Basel, Switzerland). Control PB and BM from 13 and 6 healthy donors, respectively, were used after informed consent was obtained.
Flow cytometry
Staining with monoclonal antibodies (mAbs) was performed in 100-μL aliquots of heparinized PB and BM, followed by lysis of red blood cells and fixation with 1% paraformaldehyde (PAF). For the analysis of DCs, fluorescein isothiocyanate (FITC)–conjugated lineage mAb cocktail (CD3, CD14, CD16, CD19, CD20, CD56, and CD34 for BM samples), phycoerythrin (PE)–conjugated CD123, peridinin chlorophyll protein (PerCP)–conjugated HLA-DR, and allophycocyanin (APC)–conjugated CD11c, or isotype-matched control mAbs (all from Becton Dickinson, San Jose, CA) were used. For the analysis of NK cells, staining was with the following mAbs: CD56-APC, HLA-DR-PE, CD16-PE, CD161-PE, CD3-PerCP, and CD69-FITC, or isotype-matched control mAbs (all from Becton Dickinson), and with anti-p46 mAb (IgG1, clone 9E2; kind gift from M. Colonna, Basel Institute for Immunology, Switzerland) or IgG1 control mAb, followed by FITC-conjugated goat antimouse IgG (Jackson ImmunoResearch, West Grove, PA). At least 200 000 events were acquired using fluorescence-activated cell sorting (FACS) Calibur and analysis was performed using CellQuest software (Becton Dickinson).
ELISA of cytokines
Plasma was collected from healthy donors and patients on days +7, +14, and +30 after SCT by centrifugation of heparinized PB at 3000 rpm for 10 minutes. Cytokines were measured using enzyme-linked immunosorbent assay (ELISA) reagents for human FL (kind gift from Immunex, Seattle, WA), and DuoSet ELISA reagents for human G-CSF, GM-CSF, stem cell factor (SCF), and interleukin 15 (IL-15; all from R&D Systems, Minneapolis, MN) according to the manufacturer's recommendations. ELISA detection limits were 62.5 pg/mL for FL, 31.3 pg/mL for G-CSF, 15.6 pg/mL for GM-CSF and SCF, and 1.9 pg/mL for IL-15.
NK cells from patients after SCT
NK cells were isolated from PB mononuclear cells (PBMCs) using negative selection with anti-CD3 magnetic beads followed by a positive selection with anti-CD56 beads (both from Miltenyi Biotec, Bergish Gladbach, Germany; purity > 90%) and cryopreserved. For purification of NK-cell subsets, CD56+ cells isolated as described were stained with anti-CD16-PE mAbs and sorted on a FACS Vantage SE (Becton Dickinson). Then, 10 000 to 40 000 of CD16–/low and 20 000 to 100 000 of CD16high cells were cultured for 2 weeks at 50 000 cells/mL by restimulation with phytohemagglutinin and IL-2 as described.26
NK-cell generation from CD34+ cells in vitro
CD34+ cells isolated from cord blood26 were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 5% fetal calf serum (FCS; all from Life Technologies, Grand Island, NY), and 5% normal human serum in the presence of IL-15 at 40 ng/mL, FL at 50 ng/mL (both kind gifts from Immunex), SCF at 50 ng/mL (kind gift from Amgen, Thousand Oaks, CA), and IL-2 at 12.5 ng/mL (kind gift from Novartis, Basel, Switzerland). CsA (Novartis) was added to the cultures at 10 ng to 1 μg/mL, as indicated. Half-medium change was performed weekly. After 5 weeks, CD56+ NK cells were isolated by positive selection with anti-CD56 beads (purity > 99%), and their cytotoxicity against K562 cell line was examined.
Cytotoxicity and IFN-γ expression by NK cells
For cytotoxicity studies, purified NK cells were thawed and cultured for 4 days in IMDM and 10% FCS (Life Technologies) to allow the functional recovery of cryopreserved cells. Cytotoxicity of NK cells was examined using major histocompatibility complex (MHC) class I–deficient human erythroleukemia K562 cell line as targets, at effector-target ratios ranging from 10:1 to 0.6:1. A commercial cytotoxicity assay based on lactate dehydrogenase (LDH) detection was used according to the manufacturer's instructions (Cytotox 96; Promega, Madison, WI). LDH was quantified by ELISA plate reader (Spectramax 190) and results were analyzed with Softmax software (Molecular Devices, Sunnyvale, CA). All patient- and control-derived NK-cell samples were examined simultaneously to avoid the assay-to-assay variability. Experiments were performed in quadruplicate and separate wells were set up to determine spontaneous and maximum LDH release by effector and target cells. For analysis of interferon γ (IFN-γ) production by NK-cell subsets, CD16–/low and CD16high cells were plated at 200 000 cells in 96-well plates in 200 μL IMDM containing 5% human serum. IFN-γ production was induced by overnight stimulation with 10 U/mL IL-12 and 100 ng/mL IL-18 (PeproTech, Rocky Hill, NJ) or with 100 U/mL IL-2. IFN-γ release was measured by magnetic-activated cell sorting (MACS) IFN-γ secretion assay (Mitenyi Biotec) with the addition of 32D murine cell line to prevent unspecific binding of the secreted cytokine, according to the manufacturer's instructions.
Naive T-cell activation by DCs
DCs were generated from cord blood CD34+ cells cultured in IMDM supplemented with 10% FCS in the presence of 200 U/mL GM-CSF and 100 ng/mL FL (both from Immunex) and 2.5 ng/mL tumor necrosis factor-α (TNF-α; Novartis). CsA was added to the cultures at indicated concentrations. Half-medium change was performed weekly. After 2 weeks, cells were analyzed by FACS for the presence of CD1a+ DCs. For naive T-cell activation assay, DCs were extensively washed and irradiated at 30 Gy. Naive T cells were isolated from cord blood or PB by a negative selection using anti-CD11b, anti-CD16, anti-CD19, anti-CD36, and anti-CD56 mouse mAbs and secondary goat antimouse IgG beads (T-cell isolation kit), followed by a second negative selection with anti-CD45RO and anti-CD8 beads (Miltenyi Biotec). The resulting CD4+CD45RA+ naive T cells (> 96% purity) were cultured in quadruplicate in 96 U-bottom wells (1 × 105 cells/well) with increasing numbers of irradiated DCs. Proliferative response was measured on day 5 by 3H-thymidine incorporation (1 μCi/well, [0.037 MBq/well]; Amersham Life Science, Buckingham, United Kingdom). To evaluate T-lymphocyte polarization, irradiated DCs were cocultured with naive T cells (DC/T ratio, 1:5) for 5 days. Proliferating cells were expanded with IL-2 for 8 days and subjected to stimulation with 20 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma, St Louis, MO) and 1 μM calcium ionophore A23187 (Calbiochem, La Jolla, CA) for 8 hours. Brefeldin A (5 μg/mL; Sigma) was added for the last 4 hours. Cells were fixed with 2% PAF, permeabilized with FACS buffer containing 0.1% saponin, and stained with FITC-labeled IFN-γ–specific and PE-labeled IL-4–specific mAbs (Becton Dickinson).
Statistical analysis
The nonparametric Mann-Whitney U test was used in the evaluation of the statistical differences between healthy donors and patients after SCT. Simple regression model and Spearman rank correlation test were used to analyze correlations between the cytokine plasma levels and DC and NK cell number.
Results
DC reconstitution after SCT
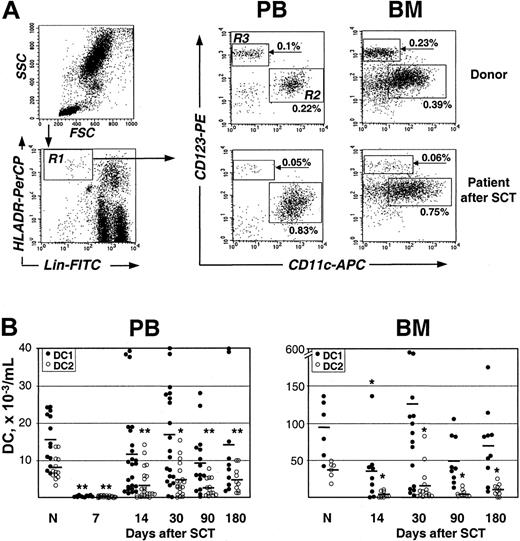
To monitor the recovery of DC subsets following allogeneic SCT, the myeloid DC1 identified as Lin–HLA-DRhiCD11c+CD123low and the lymphoid DC2 identified as Lin–HLA-DRhiCD11c–CD123+ were enumerated by 4-color FACS analysis of PB and BM cells collected from patients on days +7, +14, +30, +90, and +180 after SCT (Figure 1A-B). Similar to a previously estimated DC frequency in PB of healthy humans,4,18 the 2 DC subsets (DC1+DC2) constituted together 0.41% ± 0.04% of the nucleated cells, which was equivalent to the absolute count of 23 600 ± 220 DCs/mL PB; the corresponding values in donors' BM were 0.79% ± 0.1% or 129 000 ± 1940 DCs/mL (Table 2). After SCT, a minor DC population was detectable in PB already on day 7, although DC numbers were reduced on average about 20-fold (1100 ± 70/mL), reflecting a treatment-induced aplasia. Subsequently, DC levels rapidly increased and reached a normal percentage of nucleated cells in both PB and BM as early as on day 14. However, there was a tendency for persistent reduction of absolute DC numbers. For example, DC counts were below 10 000/mL in 7 of 20 analyzed PB samples on day 30 and remained low in 4 patients even at 6 months after SCT. Similarly, DC numbers in BM remained below 60 000/mL samples from 5 of 17 patients analyzed on day 30 and in material from 3 patients at 6 months. Interestingly, there was no difference in DC recovery patterns after the first and second SCT in 6 patients who underwent 2 consecutive transplantations from the same donor (not shown). Most remarkably, there was a change in the proportion between the 2 DC subsets after SCT in comparison with controls. In healthy individuals, the prevalence of DC1 over DC2 is 2.4 ± 0.5-fold in PB (range, 1.0-7.3) and 2.5 ± 0.3-fold in BM (range, 1.8-3.7; Figure 1; Table 2). After SCT, the DC1 and DC2 content varied highly between individual patients and, in a majority of them, there was a persistent profound reduction in DC2, which was often accompanied by a 2- to 3-fold increase in DC1. Consequently, the DC1/DC2 ratio was significantly increased up to 10.6 ± 4.3 in PB and 17.3 ± 3.5 in BM even on day 90 after SCT (Table 2). These results indicate that a rapid recovery of DC population after SCT mainly reflects reconstitution of the DC1 subset.
Reconstitution of DC1 and DC2 subpopulations after allogeneic SCT. (A) FACS analysis of DC1 and DC2 in PB and BM samples from a healthy donor and a patient 30 days after SCT (for details, see “Patients, materials, and methods”). Lin–HLA-DR+ cells (gate R1) were further analyzed for the presence of CD11c+CD123low DC1 subset (gate R2) and CD11c–CD123high DC2 subset (gate R3). DC1 and DC2 content is expressed as a percent of total nucleated cells. (B) DC1 (•) and DC2 (○) in PB and BM of individual healthy donors (N) and patients after SCT. Time after transplantation and mean values are indicated. *P < .05, **P < .005 compared with healthy controls.
Reconstitution of DC1 and DC2 subpopulations after allogeneic SCT. (A) FACS analysis of DC1 and DC2 in PB and BM samples from a healthy donor and a patient 30 days after SCT (for details, see “Patients, materials, and methods”). Lin–HLA-DR+ cells (gate R1) were further analyzed for the presence of CD11c+CD123low DC1 subset (gate R2) and CD11c–CD123high DC2 subset (gate R3). DC1 and DC2 content is expressed as a percent of total nucleated cells. (B) DC1 (•) and DC2 (○) in PB and BM of individual healthy donors (N) and patients after SCT. Time after transplantation and mean values are indicated. *P < .05, **P < .005 compared with healthy controls.
DC content after SCT correlates with levels of endogenous FL
We investigated whether a rapid recovery of DCs after SCT is related to the level of endogenous cytokines known to influence DC development. On days +7, +14, and +30, plasma concentrations of FL, GM-CSF, G-CSF, and SCF, which promote DC differentiation or mobilize DCs to circulation,21,27,28 were measured. On day 7 after SCT all patients had a severe aplasia prior to engraftment. The results of ELISA (Figure 2A) in samples collected on that day showed an average FL level of 1185 ± 812 pg/mL (range, 79-2745 pg/mL), which is well above normal levels of 14 ± 39 pg/mL.29 G-CSF was also elevated significantly to 1532 ± 551 pg/mL (range, 175-8970 pg/mL) compared with normal levels of 25.3 ± 19.7 pg/mL.30 GM-CSF concentration of 37.2 ± 11.7 pg/mL also reflected an increase above its normally unmeasurable levels.31 By day 14, when most patients recovered from leukopenia, FL and G-CSF levels remained slightly increased (171 ± 42.2 and 105 ± 28.8 pg/mL, respectively), and GM-CSF concentration fell below the ELISA detection limit. Plasma concentration of all 3 cytokines returned to normal by day 30. In contrast to FL, G-CSF, and GM-CSF, levels of SCF increased moderately from 242.1 ± 23.0 pg/mL in controls to 466.9 ± 67.5 pg/mL on day 7 and remained at this level during the study period. We found that FL levels measured on day 7 correlated with the absolute numbers of DCs on day 14 (Figure 2B) as well as day 30 after SCT (results not shown). This correlation was apparent for both DC1 and DC2 subsets (R = 0.76 and 0.72, respectively; P < .05). In contrast, G-CSF and SCF levels on day 7 correlated with DC1 and DC2 numbers on day 14 only weakly (P > .05; Figure 2C-D). Also GM-CSF concentration and DC numbers showed a poor correlation (R = 0.49, P > .05; not shown). These results suggest that the observed rapid reconstitution of DCs after SCT is due to elevated FL, rather than G-CSF, GM-CSF, or SCF during the early recovery phase.
FL plasma levels correlate with DC1/DC2 reconstitution after SCT. (A) Levels of FL (○), G-CSF (⋄), GM-CSF (▵), and SCF (□) in PB of patients on days 7, 14, and 30 after SCT, as determined by ELISA. The mean value (bars) and SEM is indicated. (B-D) Correlation between numbers of circulating DC1 and DC2 on day 14 in individual patients and day 7 plasma levels of FL, G-CSF, and SCF, respectively. Solid line shows the correlation.
FL plasma levels correlate with DC1/DC2 reconstitution after SCT. (A) Levels of FL (○), G-CSF (⋄), GM-CSF (▵), and SCF (□) in PB of patients on days 7, 14, and 30 after SCT, as determined by ELISA. The mean value (bars) and SEM is indicated. (B-D) Correlation between numbers of circulating DC1 and DC2 on day 14 in individual patients and day 7 plasma levels of FL, G-CSF, and SCF, respectively. Solid line shows the correlation.
To assess whether preferential expansion of the DC1 subset is associated with risk of GvHD, we analyzed the incidence of acute GvHD on day 30 after SCT with respect to FL levels in the preceding period of aplasia. Patients with none or mild GvHD (grade 0 or I; n = 9) had FL of 1181 ± 226 ng/mL, which was not significantly different from patients with severe GvHD (grade II-III; n = 10) who had FL of 951 ± 199 ng/mL (P > .05). Hence, there was no indication that high endogenous FL and consequently high DC1 levels aggravated the risk of acute GvHD, although investigation with a larger group of patients may be needed to exclude the association between FL and GvHD.
NK-cell reconstitution after SCT
To monitor the recovery of NK cells after allogeneic SCT, the number of CD56+CD3– cells was measured by FACS analysis of PB and BM samples at the same time intervals as the analysis of DCs. Similarly to findings with DCs, NK-cells were detectable already on day 7, although at very low numbers, and thereafter their number increased rapidly (Table 3). In PB, the frequency of CD56+CD3– cells returned to normal at 3 months (3.09% ± 0.73% versus 3.31% ± 0.74% in healthy donors), although the absolute number at that time was still below normal (8.20 ± 1.83 versus 19.46 ± 4.35 × 104/mL). The recovery of NK cells in the BM was faster than in PB.
Because FL has been implicated in the development of NK-cells from hematopoietic progenitors, NK cell numbers were related to plasma levels of FL on day 7 (Figure 2A). A weak correlation in PB on day 14 (R = 0.49, P > .05; Figure 3A) was found. The correlation with SCF levels was equally weak (R = 0.44; not shown). We then examined whether NK-cell content correlates with the concentration of IL-15, the key cytokine in NK-cell development.26 The IL-15 plasma level in healthy controls was 4.7 ± 1.4 pg/mL including 7 of 13 samples below the ELISA detection level of 1.9 pg/mL (Figure 3B). During aplasia on day 7 after SCT, IL-15 content increased to 11.1 ± 2.9 pg/mL, although it was measurable in only half the patients' samples. The correlation between NK-cell numbers on day 14 and IL-15 levels on day 7 was very poor (Figure 3C), and no correlation was also observed for later time points (not shown). These data indicate that the observed rapid recovery of NK cells is not strongly related to the levels of endogenous cytokines, neither highly increased FL nor moderately elevated SCF and IL-15.
FL and IL-15 plasma levels and NK-cell reconstitution after SCT. (A) Correlation between FL levels on day 7 and numbers of circulating NK cells on day 14 in individual patients. (B) Levels of IL-15 in PB of patients on days 7, 14, and 30 days after SCT, as determined by ELISA. The mean value (bars) and SEM is indicated. (C) Correlation between IL-15 levels on day 7 and numbers of circulating NK cells on day 14 in individual patients. Solid line shows the correlation.
FL and IL-15 plasma levels and NK-cell reconstitution after SCT. (A) Correlation between FL levels on day 7 and numbers of circulating NK cells on day 14 in individual patients. (B) Levels of IL-15 in PB of patients on days 7, 14, and 30 days after SCT, as determined by ELISA. The mean value (bars) and SEM is indicated. (C) Correlation between IL-15 levels on day 7 and numbers of circulating NK cells on day 14 in individual patients. Solid line shows the correlation.
Phenotypic and functional evaluation of NK cells after allogeneic SCT
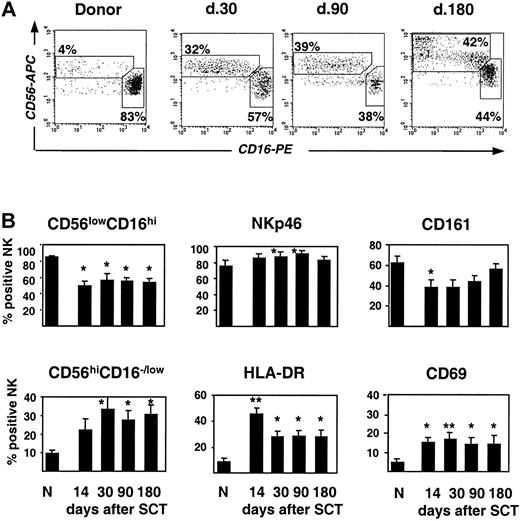
To characterize the NK-cell population that develops after allogeneic SCT, phenotypic analysis of cell-surface markers that define the maturation and activation stages of human CD56+CD3– cells was performed (Figure 4). Most remarkably, the CD56lowCD16high NK cells, which represent the predominant subset of mature cytotoxic NK cells in normal PB, were strongly reduced. Conversely, there was an accumulation of cells with a CD56highCD16–/low phenotype (Figure 4A-B). The ratio of CD56lowCD16high/CD56highCD16–/low subsets after SCT was 1.3 to 1.9, compared with 7.4 in control PB, and this strong skewing persisted for as long as 6 months in all except 2 patients. Of other analyzed markers, expression of CD161 present on both immature and mature NK cells32 was reduced, whereas NKp46, the major natural cytotoxicity receptor selectively expressed by NK cells,33 was at normal levels (Figure 4B). Expression of activation markers, CD69 and HLA-DR, was significantly increased (Figure 4B) on both CD56highCD16–/low and CD56lowCD16high cell subsets (not shown). Thus, despite rapid numerical recovery of NK cells, the phenotypic profile reflects distinct abnormalities in NK-cell differentiation after SCT.
Phenotypic characteristics of NK cells after allogeneic SCT. (A) FACS profile of CD56highCD16–/low and CD56lowCD16high subpopulations of NK cells in a patient after SCT compared with the healthy donor. (B) Percentage of CD56highCD16–/low and CD56lowCD16high NK cell subpopulations and expression of NK cell markers NKp46, CD161, HLA-DR, and CD69 in patients after SCT and healthy donors (N) is shown (mean ± SEM). Time after transplantation is indicated. *P < .05, **P < .005 compared with healthy donors.
Phenotypic characteristics of NK cells after allogeneic SCT. (A) FACS profile of CD56highCD16–/low and CD56lowCD16high subpopulations of NK cells in a patient after SCT compared with the healthy donor. (B) Percentage of CD56highCD16–/low and CD56lowCD16high NK cell subpopulations and expression of NK cell markers NKp46, CD161, HLA-DR, and CD69 in patients after SCT and healthy donors (N) is shown (mean ± SEM). Time after transplantation is indicated. *P < .05, **P < .005 compared with healthy donors.
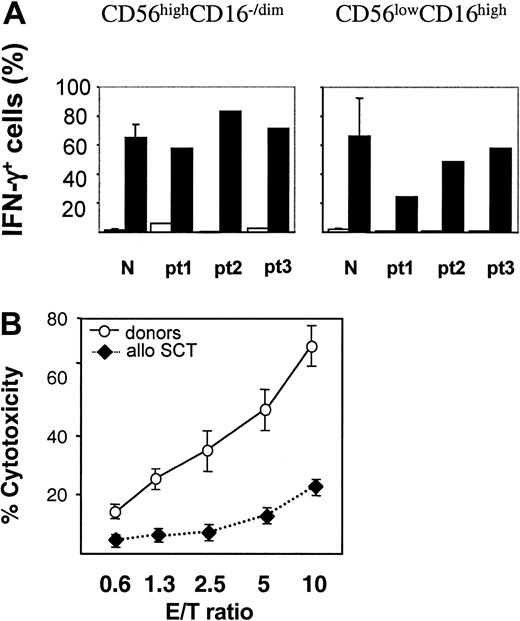
To examine the functional properties of the NK-cell compartment during early recovery after SCT, CD56highCD16–/low and CD56lowCD16high NK-cell subsets were purified from patients' PB on day 30 and their ability to produce IFN-γ was examined in response to specific stimulation with IL-12 and IL-18. Abundant IFN-γ production was seen with the CD16–/low subset, with 55% to 85% IFN-γ+ cells, whereas stimulation of CD16high cells resulted in 25% to 55% of IFN-γ+ cells (Figure 5A). Therefore, CD16–/low cells present in increased proportions in the early posttransplantation period have the ability to readily respond to the cytokine stimulus. Subsequently, we analyzed the capacity of NK cells to kill MHC class I–deficient target cells. CD56+ NK cells were purified from PB on day 14 or 30 after SCT and their cytotoxicity was assessed against K562 cells (Figure 5B). The killing potential of patient-derived NK cells was reduced 3- to 4-fold, compared with NK cells isolated from healthy donors. This significant difference may be related to the reduced content of highly cytotoxic CD56lowCD16high subset within the NK-cell population (Figure 4).
IFN-γ production and cytotoxicity of NK cells from patients after SCT. (A) IFN-γ production by NK-cell subsets. CD16–/low and CD16high cells were purified, expanded, and stimulated with IL-2 (□) or IL-12 plus IL-18 (▪). Frequency of IFN-γ+ NK cells was determined by MACS IFN-γ secretion assay as described in “Patients, materials, and methods.” Results obtained with healthy donors (n = 3) and 3 patients (pt) after SCT are shown. (B) Cytotoxicity of NK cells against K562 target cells. NK cells were purified from PB of healthy donors (○) or patients after allogeneic SCT (♦) collected at 14 and 30 days after SCT. Cytotoxicity was measured as LDH released from lysed K562 cells (“Patients, materials, and methods”). Results obtained with 3 healthy donors and 5 patients after SCT are shown (mean ± SEM).
IFN-γ production and cytotoxicity of NK cells from patients after SCT. (A) IFN-γ production by NK-cell subsets. CD16–/low and CD16high cells were purified, expanded, and stimulated with IL-2 (□) or IL-12 plus IL-18 (▪). Frequency of IFN-γ+ NK cells was determined by MACS IFN-γ secretion assay as described in “Patients, materials, and methods.” Results obtained with healthy donors (n = 3) and 3 patients (pt) after SCT are shown. (B) Cytotoxicity of NK cells against K562 target cells. NK cells were purified from PB of healthy donors (○) or patients after allogeneic SCT (♦) collected at 14 and 30 days after SCT. Cytotoxicity was measured as LDH released from lysed K562 cells (“Patients, materials, and methods”). Results obtained with 3 healthy donors and 5 patients after SCT are shown (mean ± SEM).
Effect of CsA on differentiation of NK cells and DCs in vitro
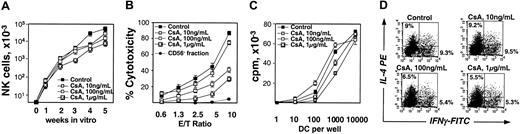
Considering the possibility that immunosuppressive treatment with CsA has an influence on NK-cell and DC development from engrafted donor cells, CsA effect was examined in vitro under conditions leading to NK-cell and DC differentiation from CD34+ progenitors.15,21 When CsA was added to cultures containing FL, SCF, and IL-15, a dose-dependent decrease in the output of CD56+CD3– cells was observed (Figure 6A), with a maximum reduction by CsA at 1 μg/mL (6.6-fold, P = .017). After 5 weeks of culture, CD56+ NK cells were purified and used in a cytotoxicity assay against K562 cells. As shown in Figure 6B, NK cells generated in the presence of CsA were less cytotoxic than control NK cells and this deficiency in the killing ability was proportional to the CsA dose. These results are reminiscent of the low cytotoxicity of NK cells after SCT (Figure 5B) and suggest that also when applied therapeutically, CsA may delay NK-cell reconstitution and impair the killing potential of NK cells.
Effect of CsA on NK cells and DCs generated in vitro from CD34+ progenitors. (A) NK-cell generation in the absence or presence of the increasing concentrations of CsA for 5 weeks. CD56+CD3– NK-cell content was determined weekly by FACS. A summary of 3 independent experiments performed in duplicate is shown (mean ± SEM). (B) Cytotoxicity of in vitro–generated NK cells against K562 target cells. NK cells generated after 5 weeks of the culture as in panel A were isolated and their cytotoxicity at increasing effector-target (E/T) ratio was measured as LDH released from lysed cells. CD56– (non-NK) cells obtained from the control culture after NK-cell purification were used as a negative control. A summary of 4 independent experiments is shown (mean ± SEM). (C) Proliferative response of naive T cells stimulated with DCs generated in vitro in the presence of increasing concentrations of CsA. DCs were used in allogeneic mixed lymphocyte reaction and a proliferative response of naive CD4+ T cells was measured by 3H-thymidine uptake (cpm). A summary of 3 independent experiments is shown (mean ± SEM). (D) Naive T-cell polarization by in vitro generated DCs. DCs obtained as in panel C were cocultured with naive CD4+CD45RA+ T cells, and intracellular staining with IL-4– and IFN-γ–specific mAbs was performed. Representative FACS results of 1 of 3 experiments are shown.
Effect of CsA on NK cells and DCs generated in vitro from CD34+ progenitors. (A) NK-cell generation in the absence or presence of the increasing concentrations of CsA for 5 weeks. CD56+CD3– NK-cell content was determined weekly by FACS. A summary of 3 independent experiments performed in duplicate is shown (mean ± SEM). (B) Cytotoxicity of in vitro–generated NK cells against K562 target cells. NK cells generated after 5 weeks of the culture as in panel A were isolated and their cytotoxicity at increasing effector-target (E/T) ratio was measured as LDH released from lysed cells. CD56– (non-NK) cells obtained from the control culture after NK-cell purification were used as a negative control. A summary of 4 independent experiments is shown (mean ± SEM). (C) Proliferative response of naive T cells stimulated with DCs generated in vitro in the presence of increasing concentrations of CsA. DCs were used in allogeneic mixed lymphocyte reaction and a proliferative response of naive CD4+ T cells was measured by 3H-thymidine uptake (cpm). A summary of 3 independent experiments is shown (mean ± SEM). (D) Naive T-cell polarization by in vitro generated DCs. DCs obtained as in panel C were cocultured with naive CD4+CD45RA+ T cells, and intracellular staining with IL-4– and IFN-γ–specific mAbs was performed. Representative FACS results of 1 of 3 experiments are shown.
When CsA was added to cultures containing FL, GM-CSF, and TNF-α, the output of CD1a+ DCs was similar to cultures without CsA (not shown). The ability of DCs generated in the presence of CsA to induce naive T-cell proliferation varied, in particular at limiting DC numbers, but no CsA dose dependence was noted (Figure 6C). The potential of DCs to polarize naive T cells toward IL-4 or IFN-γ production was retained, although the frequency of cytokine-producing cells was reduced by about 40% already at 100 ng/mL CsA, corresponding to physiologic concentrations of CsA in patients under immunosuppression (Figure 6D). These data indicate the lineage-specificity of CsA effects, which involve suppression of growth and function of NK cells, but are less pronounced with respect to proliferation and capacity of DCs for T-cell priming.
Discussion
The process of immune recovery after SCT is influenced by a multitude of parameters, which include the patient's clinical history as well as treatment-related factors such as conditioning regimen, cellular composition of the graft, and immunosuppressive therapy after grafting.34,35 Cytokine expression profile after SCT represents an intrinsic parameter that can influence the regeneration of immunocompetent cells from hematopoietic progenitors. In this study we investigated the role of endogenous cytokines in the recovery of DC and NK-cell compartments, and we demonstrate that reconstitution of DCs correlates with strongly increased levels of FL in the early period after SCT period.
The recovery of DCs and NK cells after grafting is very rapid. As soon as 14 days after SCT both cell types circulate in measurable numbers in the PB and can be detected in the BM of graft recipients. According to recent chimerism studies, the major proportion of DC and NK cells is donor derived3,5 and, at this early time point, may represent a mixture of cells transferred with the graft and those developing from the newly engrafted hematopoietic progenitors. The process of recovery of bone marrow function is tightly regulated and involves complex cell-cell interactions in the enriched cytokine milieu within the environment provided by BM stroma. A coordinate pattern of cytokine release in response to treatment-induced pancytopenia defines links between cytokine networks and multilineage hematopoietic reconstitution. Thus, a transient increase in circulating G-CSF, GM-CSF, IL-6, and IL-8 has been linked to neutrophil recovery,36 and thrombopoietin to platelet production after SCT.37 We now demonstrate that FL, present in highly elevated concentrations in a vast majority of patients during the first 14 days after SCT, correlates with early recovery of the DC lineage of both lymphoid and myeloid origin. FL was first discovered by the ability to stimulate the proliferation of hematopoietic progenitor cells.38 It shows specificity toward the precursors of the DC lineage, as evidenced by strong and selective expansion of circulating and tissue DCs following administration of FL to mice and humans.17,18,21 Moreover, flt3+ but not flt3– precursors can differentiate along the DC pathway in vivo.39 FL treatment increases numbers of CD11c+ DC1 about 4 times more efficiently than those of CD11c– DC2. Interestingly, during the period of DC regeneration after SCT, the DC1 subset prevails to the same extent over DC2, suggesting that the preferential expansion of DC1 lineage is a response to high endogenous FL levels. This prevalence of DC1 occurs despite a simultaneous increase of G-CSF, the cytokine that selectively mobilizes the DC2 subset into blood.19,21,40 It is also possible that FL, which rises only transiently during the period of aplasia, is not available after stem cell engraftment long enough for the DC2 subset to develop.15 The frequency of DC1 and DC2 correlates poorly with levels of endogenous G-CSF, GM-CSF, and SCF, the cytokines that enhance DC generation in vitro but are less potent when administered in vivo,17 arguing further for a dominance of FL-specific effects on the DC regeneration in the early period following transplantation.
In vitro effects of FL on the development of NK cells from CD34+ progenitors, as well as NK-cell deficiency in FL–/– mice provided arguments for a role of FL in NK-cell development.13,16 However, in vivo administration of FL does not significantly alter the number of circulating lymphocytes.18 Accordingly, a correlation between high FL levels after SCT with NK-cell numbers was much weaker than with DCs. We also found no correlation of circulating NK-cell numbers with IL-15, the cytokine essential for human NK-cell development in vitro and in vivo,16,41 but found only moderately elevated levels in a proportion of patients receiving transplants. Although local stimulatory effects of FL and IL-15 produced by BM stroma should be taken into consideration, other microenvironmental factors are likely to influence the regeneration of the NK-cell compartment.
Little is known so far about effects of immunosuppressive therapy with CsA on cell lineages other than T cells. Recently, a moderate inhibition of DC functions was demonstrated following CsA treatment of DCs isolated from human PB or derived from monocytes.42,43 We show that DCs generated in vitro from CD34+ precursors in the presence of CsA have a reduced capacity to prime naive T cells for production of IFN-γ and IL-4, suggesting direct effects of CsA on differentiation and immunomodulatory function of DCs. The immunosuppressive treatment is also likely to interfere indirectly with the generation of DCs by partly preventing the up-regulation of FL during aplasia.24 Further, we show that CsA significantly delays the differentiation and lowers cytotoxicity of NK cells generated from CD34+ progenitors in vitro, suggesting that CsA used for prevention of GvHD may have an impact on the functional recovery of NK-cell compartment. Indeed, cytotoxicity of NK cells after allogeneic SCT was impaired compared with healthy controls. Inadequate function of NK cells from the early time point after transplantation may be related to the disproportional 5-fold increase in poorly cytotoxic CD56highCD16–/low cells over highly cytotoxic CD56dimCD16high NK-cell population, which is in agreement with earlier observations.44 It is possible that high endogenous FL contributes to this skewing of NK-cell subsets during the immune recovery, because NK cells generated from CD34+ cells in response to FL in vitro are predominantly CD56high type.16 It remains unknown whether CD56highCD16–/low population represents NK cells that did not complete the process of maturation,10 but we show that already early after SCT, these cells have acquired the ability to respond to specific stimuli and abundantly produce the IFN-γ.
Because reconstitution of a diversified T-cell repertoire starts approximately 6 months after SCT and it may take years before T-cell immunity is restored,1,2 the rapid expansion of donor-derived DCs and NK cells has particular implications for the functional immune recovery in the early period after SCT. Interactions between DCs and T cells and a more recently established cross talk between DCs and NK cells45 underlie the main immunologic responses important for induction of tolerance to prevent GvHD and for an antitumor effect to prevent relapse. In animal models, FL generates an effective response to tumors by promoting the DC-NK cell interactions46,47 and was reported to induce tolerance and reduce GvHD by expanding the CD8α+ subset of host DCs.48 The relevance of FL-mediated DC expansion for the allograft reactivity in humans remains unclear. Based on results of this study, high FL levels and the preferential expansion of DC1, having a potential to elicit immunogenic TH1-type responses,9 was not associated with high incidence of acute GvHD. Similarly, it has been reported previously that DC numbers in patients with acute GvHD are usually below normal values.4 Hence, there is so far no evidence that high endogenous FL and consequently high DC1 levels may aggravate the risk of acute GvHD. In mice, treatment with FL enhances protective immunity against pathogens,49,50 possibly related to efficient capturing of microbial antigens by tissueresident myeloid DC1, and may be important for reducing the risk of opportunistic infections after SCT. It is conceivable that clinical application of FL to achieve concentrations higher than endogenous in patients who undergo SCT may have multiple beneficial effects by accelerating the hematopoietic and the immune recovery.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-04-1200.
Supported by grants from the Swiss National Science Foundation (3100-067072.01 and 4046-058689) and Swiss Cancer League KFS-00951-09-1999.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank S. Sendelov for technical help; S. D. Lyman for FL-related reagents; M. Colonna and A. Bouchon for anti-NKp46 mAbs; W. Holzgreve, D. Surbek, and S. Heinzl for cord blood samples; D. Heim for help with patients' material; and C. P. Kalberer for critically reading the manuscript.