Abstract
The MLL (mixed lineage leukemia) gene forms chimeric fusions with a diverse set of partner genes as a consequence of chromosome translocations in leukemia. In several fusion partners, a transcriptional activation domain appears to be essential for conferring leukemogenic capacity on MLL protein. Other fusion partners, however, lack such domains. Here we show that gephyrin (GPHN), a neuronal receptor assembly protein and rare fusion partner of MLL in leukemia, has the capacity as an MLL-GPHN chimera to transform hematopoietic progenitors, despite lack of transcriptional activity. A small 15–amino acid tubulin-binding domain of GPHN is necessary and sufficient for this activity in vitro and in vivo. This domain also confers oligomerization capacity on MLL protein, suggesting that such activity may contribute critically to leukemogenesis. The transduction of MLL-GPHN into hematopoietic progenitor cells caused myeloid and lymphoid lineage leukemias in mice, suggesting that MLL-GPHN can target multipotent progenitor cells. Our results, and other recent data, provide a mechanism for oncogenic conversion of MLL by fusion partners encoding cytoplasmic proteins.
Introduction
Chromosome translocations involving 11q23 fuse the MLL (mixed lineage leukemia) gene to almost 40 identified partner genes to create chimeric genes and hybrid proteins.1,2 This type of chromosomal rearrangement is particularly prevalent in infant leukemia,3 but it is also common in secondary leukemias associated with previous exposure to topoisomerase-inhibiting epipodophyllotoxins or anthracyclins.4 Most patients with MLL gene rearrangement have a poor prognosis. This, along with brief latency after formation of MLL gene fusion in utero5 or following exposure to therapeutic topoisomerase-II inhibitors,6 suggests that MLL fusion proteins may have powerful transforming functions in hematopoietic progenitors. MLL is the mammalian equivalent of Drosophila trithorax, which is a critical developmental regulator of Homeobox complex (HOX-C) gene expression. Murine MLL encodes a histone methyltransferase that impacts on chromatin structure and transcriptional regulation of HOX and other genes involved in hematopoiesis.7,8 The conundrum has been to explain how, in leukemia, so many MLL partner genes encoding both nuclear and cytoplasmic proteins with diverse functions can convert MLL to a chimeric oncogene. Any explanation would need to accommodate the fact that MLL internal duplications can also be leukemogenic but MLL truncation alone is not.
Previous analyses of several MLL fusion partners identified in leukemic cells, such as ENL, ELL, AF10, AFX, and FKHRL1, showed that the minimum region of fusion partner required for hematopoietic cell transformation contains a transcriptional activation domain.9-14 Further evidence comes from studies in which the transcriptional activation domain of herpes simplex virus (HSV) VP16 conferred on MLL competence to transform murine primary bone marrow cells.15 These data support the notion that a transcriptional domain may be an obligatory requirement for transformation by MLL fusion protein.9,13 However, knock-in of an MLL–β-galactosidase (LacZ) gene also induced leukemia, albeit with long latency.16 LacZ itself has not been reported to have any transcriptional activity, but one possibly relevant feature of LacZ is its capacity to form tetramers.17 This suggested the possibility that 3′ protein sequences contributed by some of MLL's fusion partners might provide regions that facilitate oligomerization of MLL, which, in turn, alters 5′ MLL protein function, interaction with DNA for example, in such a way as to be transforming.
MLL-gephyrin was cloned from acute myeloid leukemia (AML) with t(11;14)(q23;q24).18,19 Gephyrin (GPHN; from the Greek word for “bridge”) is a neuronal receptor assembly protein that links membrane-associated receptor molecules to cytoskeletal microfilaments.20,21 Exon 14 of GPHN22 encodes 15 amino acids (VQSRCSSKENILRAS) with homology to the C-terminal–repeat motif of microtubule-associated proteins tau and microtubule-associated protein 2 (MAP2). These latter motifs promote tubulin polymerization23 and self-oligomerization.24 It is considered likely that GPHN exon 14 (ex14) domain exercises a similar cross-linking function.25 We used MLL-GPHN to elucidate its function as an MLL fusion partner and show here that the small (15 amino acid) oligomerization domain encoded by exon 14 of GPHN is both necessary and sufficient to give the MLL fusion protein transformation capability. These results highlight acquisition of oligomerization-encoding sequences as a likely important mechanism for the oncogenic conversion of the MLL gene by some of its leukemia-associated partner genes.
Materials and methods
Retroviral vectors
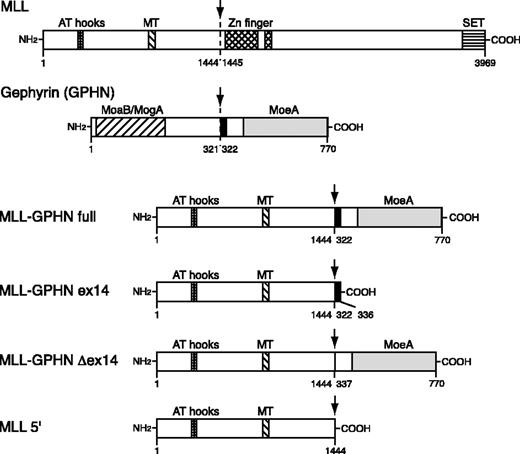
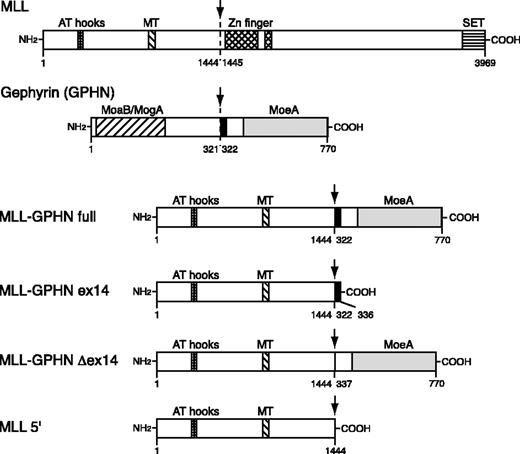
The domain structure of MLL, GPHN, and the chimeric protein in leukemia (MLL-GPHN full) is depicted in Figure 1. For functional studies we used 4 distinct constructs (Figure 1): the full-length MLL-GPHN; MLL-GPHN ex14, which had exon 14 only of GPHN fused to 5′ MLL sequences; MLL-GPHN Δex14 lacking exon 14; and MLL 5′ with no GPHN sequences (ie, truncated MLL). Retroviral constructs were made by cloning the MLL-GPHN cDNA sequence and its mutants into murine stem cell virus 2.1 (MSCV2.1) vector with the addition of an N-terminal Flag epitope tag. MLL-GPHN full construct encodes chimeric protein detected in a patient's sample18 in which the N-terminal portion of MLL (amino acids [aa's] 1-1444) is fused to the C-terminal portion of GPHN (aa's 322-770; Figure 1). In the MLL-GPHN ex14 construct, aa's 322-336 of GPHN coded by exon 14 are fused to the same N-terminal portion of MLL. In the MLL-GPHN Δex14 construct, the 15 amino acids coded by exon 14 are removed from full-length MLL-GPHN. The MLL 5′ construct contains only the MLL sequence 5′ of the translocation breakpoint.
Schematic of MLL-GPHN and its mutants used in this study. The chimeric gene in leukemia is MLL-GPHN full. MT indicates DNA methyltransferase homology domain; SET, SET domain; MoaB, MogA, and MoeA, regions in GPHN with sequence similarities to the respective Escherichia coli proteins involved in molybdenum cofactor biosynthesis. Arrows indicate the fusion site. Numbers refer to amino acid positions in wild-type MLL or GPHN. The black bar indicates the tubulin-binding site of GPHN.
Schematic of MLL-GPHN and its mutants used in this study. The chimeric gene in leukemia is MLL-GPHN full. MT indicates DNA methyltransferase homology domain; SET, SET domain; MoaB, MogA, and MoeA, regions in GPHN with sequence similarities to the respective Escherichia coli proteins involved in molybdenum cofactor biosynthesis. Arrows indicate the fusion site. Numbers refer to amino acid positions in wild-type MLL or GPHN. The black bar indicates the tubulin-binding site of GPHN.
Retroviral transduction of mouse primary hematopoietic cells
Transduction of murine hematopoietic progenitor cells was performed as described previously26 with some modifications. Four-week-old Balb/c mice were injected intravenously with 5-fluorouracil (5-FU; 150 mg/kg) and 5 days later bone marrow cells were harvested. Bone marrow cells were enriched for immature cells by immunomagnetic depletion with a cocktail of antibodies (CD5, B220, Gr-1, Mac-1, 7-4, TER119) directed against mature myeloid, lymphoid, and erythroid antigens (Stem Cell Technologies, Vancouver, BC, Canada). Retroviral supernatants were produced by transient transfection of Phoenix packaging cells with MSCV constructs. Chimeric gene expression was determined by Western blot analysis using anti-Flag monoclonal antibody (Sigma, Poole, United Kingdom) in these cells. Lineage-depleted bone marrow (Lin– BM) cells were infected with retrovirus by standard methods. After 2 serial infections, infected cells were plated into methylcellulose cultures.
Methylcellulose colony replating assay
Retrovirally infected Lin– BM cells (1 × 104) were plated in 1% methylcellulose (Stem Cell Technologies) supplemented with 10 ng/mL each of murine interleukin-3 (IL-3), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and 20 ng/mL stem cell factor (SCF) in the presence of 1 mg/mL G418. After 7 days of culture, colonies were counted and 1 × 104 of single-cell suspensions of G418-resistant colonies were replated into methylcellulose supplemented with the same growth factors, without G418. Single-cell suspensions were also expanded in RPMI1640 medium containing 20% fetal calf serum (FCS) and WEHI-conditioned medium. Further plating was repeated every 7 days.
Injection of transduced cells into NOD/SCID mice
Four-week-old nonirradiated nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were injected intravenously (tail vain) with 106 MLL-GPHN full– or MLL-GPHN ex14–transduced Lin– BM cells from early passage of liquid cultures derived from first-round methylcellulose colonies.12 Mice were observed on a daily basis for the onset of symptoms and culled when morbid.
Subcellular localization of MLL-GPHN
NIH3T3 cells were transiently transfected with constructs expressing the MLL-GPHN fusion protein and its mutants, which were fused in-frame with enhanced green fluorescent protein (EGFP; N-terminal). Thirty-six hours after transfection, cells were fixed and counterstained with TO-PRO-3 (Molecular Probes, Eugene, OR) and observed with confocal microscopy.
In vivo cross-linking experiments
MLL(exon 5-8)–GPHN and its mutant constructs, which contain MLL sequences 3′ downstream of a BamHI site within exon 5 of MLL (corresponding to MLL aa's 1251-1444) and full-length or mutant GPHN sequences, were subcloned into pcDNA3 with N-terminal Flag tag. We excluded exons 1 to 4 of MLL for better resolution in Western blotting. The 293T cells were transiently transfected with these expression vectors and analyzed for oligomerization capacity 36 hours after transfection as previously described27 with some modifications. Briefly, cells were washed with phosphate-buffered saline (PBS) and incubated for 1 hour at room temperature in PBS with 5 mM DTBP (dimethyl 3,3′-dithiobispropionamidate-2HCl; Sigma). Cells were washed twice with PBS and then cell extracts were prepared by lysing cells with cell lysis buffer (150 mM NaCl, 1% TritonX, 2 mM EDTA [ethylenediaminetetraacetic acid], 5 mM NaF, aprotinin and leupeptin 10 μg/mL, pepstatin 2 μg/mL, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 100 mM Tris-Cl). Protein samples were separated on polyacrylamide gels in nonreducing and reducing conditions and blotted on polyvinylidene fluoride membrane (Immobilon-P; Millipore, Bedford, MA). Membranes were probed with monoclonal antibody against Flag epitope (Sigma).
Luciferase assay
DNA binding moiety of GAL4 protein (aa's 1-147) was fused in-frame to GPHN full (aa's 322-770, the portion involved in MLL fusion), its mutant GPHN ex14 (aa's 322-336), and the C-terminal portion of ENL (aa's 431-559) using the expression vector pGALO. The GAL4(RE)5-tkluc reporter was as previously described.28 Full-length histone-deacetylase 1 (HDAC1) and nuclear receptor corepressor (NcoR) fused to GAL4 DNA binding domain (DBD) was used as repression control. Transient transfections of 293T cells were carried out using Effectene (Qiagen, Crawley, United Kingdom) with 100 ng of GAL4(RE)5-tkluc reporter plasmid, 300 ng of various GAL4(DBD)–X expression vector (X represents different genes fused to GAL4 as shown in Figure 6), and pRL-CMV Renilla vector (Promega, Southampton, United Kingdom) as an internal control. Reporter assay was performed using Dual-Luciferase Reporter Assay System (Promega) after 36 hours after transfection. Experiments were repeated at least 3 times. Western blot analysis confirmed the expression of GAL4 constructs.
GPHN lack intrinsic transcriptional activation and repression properties. Five tandemly arrayed GAL4 recognition sites were introduced upstream of a HSV thymidine kinase (TK) promoter (A) and used to assess the effects of GPHN on transcriptional activity. X represents different genes shown in B and C. GAL4(DBD)–GPHN full and GAL4(DBD)–GPHN ex14 lack transcriptional activation (B) or repression activities (C) in transient assays. The results are presented as the fold activation or repression relative to the empty expression plasmid. The result is the average of normalized luciferase values of at least 3 independent experiments.
GPHN lack intrinsic transcriptional activation and repression properties. Five tandemly arrayed GAL4 recognition sites were introduced upstream of a HSV thymidine kinase (TK) promoter (A) and used to assess the effects of GPHN on transcriptional activity. X represents different genes shown in B and C. GAL4(DBD)–GPHN full and GAL4(DBD)–GPHN ex14 lack transcriptional activation (B) or repression activities (C) in transient assays. The results are presented as the fold activation or repression relative to the empty expression plasmid. The result is the average of normalized luciferase values of at least 3 independent experiments.
Results
MLL-GPHN is a nuclear protein
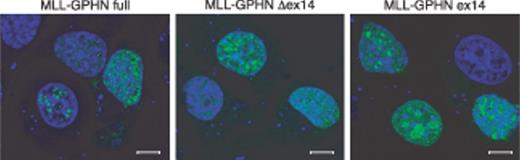
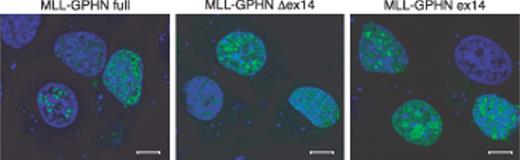
Several studies have shown nuclear localization of normal and chimeric MLL proteins with a punctate distribution.29,30 GPHN itself is a cytoplasmic protein and in neuronal cells is located under the synaptic membrane.31 Transduced GPHN in nonneuronal cells also is located in cytoplasm with the formation of aggregates.32 MLL-GPHN fused in-frame with EGFP at the N-terminal was transiently expressed in NIH3T3 cells. MLL-GPHN full-length as well as its mutants, MLL-GPHN Δex14 and MLL-GPHN ex14, localized to the nucleus with aggregates or spots of variable size and number (Figure 2), indicating that fusion to the GPHN encoding sequence does not alter the nuclear localization of the MLL protein.
Nuclear localization of MLL-GPHN chimeric protein and its mutants. EGFP-tagged MLL-GPHN and its mutants were transiently transfected in NIH3T3 cells and counterstained with TO-PRO-3. Scale bars indicate 8 μm in length.
Nuclear localization of MLL-GPHN chimeric protein and its mutants. EGFP-tagged MLL-GPHN and its mutants were transiently transfected in NIH3T3 cells and counterstained with TO-PRO-3. Scale bars indicate 8 μm in length.
MLL-GPHN can immortalize mouse hematopoietic progenitor cells in vitro and the tubulin-binding domain of GPHN (exon 14) is sufficient for transformation
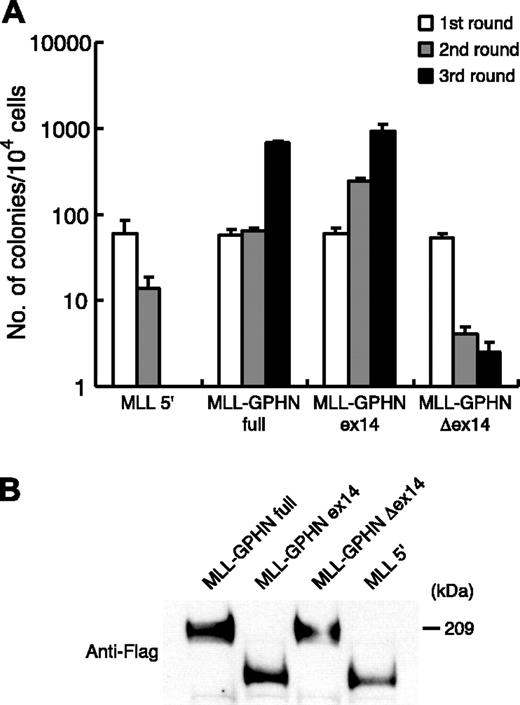
A serial methylcellulose replating assay was used to investigate the transforming effects of MLL-GPHN in murine hematopoietic progenitor cells.26 Colonies form in tertiary rounds of plating only if a cell with self-renewing capacities has been immortalized. Nontransformed cells exhaust their proliferative potential and terminally differentiate. Primary murine Lin– BM cells enriched in hematopoietic progenitors by depletion of terminally differentiated cells were transduced with retroviruses encoding MLL-GPHN full-length, its mutants, or MLL 5′ (Figure 1). Expression of MLL-GPHN and its mutants were confirmed by reverse transcriptase–polymerase chain reaction (RT-PCR; data not shown). First-round plating of transduced cells yielded similar numbers of G418-resistant colonies for all 4 constructs, with comparable transduction efficiencies between the constructs. However, significant differences were observed between the constructs in third-round plating (Figure 3A). Lin– BM cells transduced with N-terminal MLL had exhausted their self-renewal potential by the third round and cells with the MLL-GPHN Δex14 mutant had residual low-level clonogenicity. In contrast, Lin– BM cells transduced with MLL-GPHN full-length and MLL-GPHN ex14 only continued to grow, indicative of self-renewal capacity. The experiments were repeated at least 3 times with similar results. All constructs were expressed at similar levels in transduced Phoenix cells (Figure 3B).
In vitro proliferative effect of MLL-GPHN and its mutants. (A) Forced expression of MLL-GPHN full and ex14 immortalize murine hematopoietic progenitors. The bars represent number of colonies generated per 104 cells in first, second, and third rounds of plating in methylcellulose. The error bars represent the mean ± SD of triplicate samples from a representative experiment. (B) Detection of MLL fusion protein expression in Phoenix cells by Western blot analysis.
In vitro proliferative effect of MLL-GPHN and its mutants. (A) Forced expression of MLL-GPHN full and ex14 immortalize murine hematopoietic progenitors. The bars represent number of colonies generated per 104 cells in first, second, and third rounds of plating in methylcellulose. The error bars represent the mean ± SD of triplicate samples from a representative experiment. (B) Detection of MLL fusion protein expression in Phoenix cells by Western blot analysis.
Cells harvested from first-round methylcellulose culture were also propagated in liquid culture. In accord with the results of the replating assay, cells transduced with MLL-GPHN full-length and MLL-GPHN ex14 (but not MLL-GPHN Δex14 or MLL 5′) could be maintained in long-term culture (> 6 months). However, these immortalized cells remained growth factor dependent and could not grow without WEHI medium or IL-3. Cells in early passage liquid culture showed features consistent with immature progenitors, immunophenotyping of these cells with MLL-GPHN full-length and MLL-GPHN ex14 being c-kit (CD117) positive, Sca-1 positive, and CD34 positive but negative for other lineage-specific markers (Mac-1, Gr-1, B220, CD19, and CD3), reflecting arrest at an early stage of hematopoietic differentiation.
MLL-GPHN–transduced cells induce leukemia in NOD/SCID mice
The progenitor cells immortalized by MLL-GPHN were injected into NOD/SCID mice to evaluate leukemogenic potential.12 Around 80% of mice injected with 106 MLL-GPHN full-length or MLL-GPHN ex14 cells died within 6 months (Figure 4A). Peripheral blood and bone marrow displayed hyperleukocytosis with myeloblasts/monocytes or lymphoblasts (Figure 4B). Morphologic characteristics of leukemic cells from full-length MLL-GPHN–transduced leukemia mice and MLL-GPHN ex14 leukemia mice were very similar, with myeloblasts with clear azurophilic granule and Auer body dominating in the bone marrow in AML (Figure 4B). Expression of MLL-GPHN in leukemic cells was confirmed by RT-PCR using bone marrow cells (data not shown). All injected mice had significant hepatosplenomegaly (Table 1), with a massive infiltration of leukemic blasts observed by histologic analysis. Leukemic blasts occupying the bone marrow showed a myeloid phenotype expressing Mac-1/Gr-1 in cells from 14 mice (8 full-length and 6 ex14), a lymphoid phenotype expressing B220/CD19 in 1 mouse (full-length; Figure 4B-C), and a mixed-lineage phenotype showing both Mac-1/Gr-1 positivity and B220 positivity in 2 mice (1 full-length and 1 ex14). These partially differentiated phenotypes contrast to the more primitive or stem cell phenotype (CD34+/Sca-1+/Lin–) of the cells propagated in vitro. The difference may reflect the provision of differentiation signals in vivo and parallels the phenotypic hierarchy observed in human AML.33
MLL-GPHN immortalized cells cause leukemia in vivo. (A) Survival curves of mice (n ≥ 10) injected with immortalized cells by MLL-GPHN full-length, MLL-GPHN ex 14, or mock-injected (control) mice. (B) May-Grünwald/Giemsa–stained cells from bone marrow of injected mice (original magnification, × 600). Massive infiltration of leukemic cells in bone marrow is observed, which also appears in peripheral blood. (C) Fluorescence-activated cell sorter analysis of leukemic cells. Myeloid lineage-specific markers are apparent in most of the mice, although lymphoid lineage-specific markers are detected in some mice.
MLL-GPHN immortalized cells cause leukemia in vivo. (A) Survival curves of mice (n ≥ 10) injected with immortalized cells by MLL-GPHN full-length, MLL-GPHN ex 14, or mock-injected (control) mice. (B) May-Grünwald/Giemsa–stained cells from bone marrow of injected mice (original magnification, × 600). Massive infiltration of leukemic cells in bone marrow is observed, which also appears in peripheral blood. (C) Fluorescence-activated cell sorter analysis of leukemic cells. Myeloid lineage-specific markers are apparent in most of the mice, although lymphoid lineage-specific markers are detected in some mice.
MLL-GPHN forms oligomers
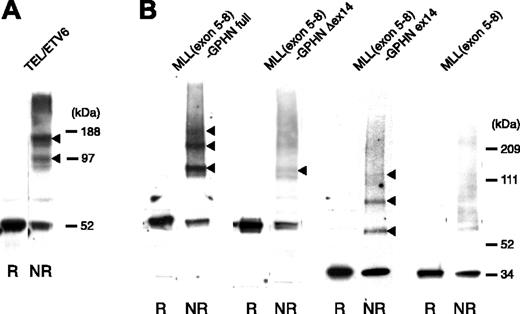
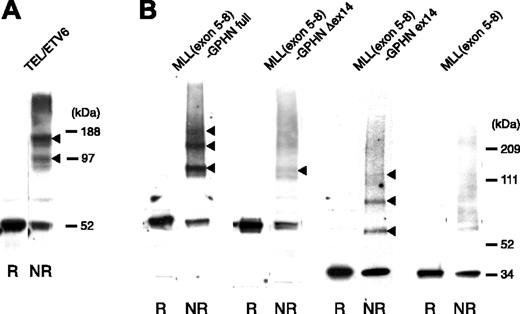
We next evaluated whether MLL-GPHN oligomerized and the dependence of any such activity on GPHN exon 14. The 293T cells were transiently transfected with MLL(exon 5-8)–GPHN or its mutant constructs and were treated with the reversible cross-linking agent DTBP followed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions (to preserve cross-linking and to detect oligomeric complexes) and reducing conditions (to revert cross-linking).
A full-length TEL/ETV6 gene construct was used for positive control, as TEL has strong oligomerization capacity34 (Figure 5A). As shown in Figure 5B, MLL(exon 5-8)–GPHN full-length showed multiple retarded bands in nonreducing conditions approximating to the size of dimers and tetramers, indicating strong oligomerization capacity. MLL(exon 5-8)–GPHN ex14 also formed dimers and tetramers (Figure 5B arrows), albeit not as strongly as MLL(exon 5-8)–GPHN full-length. MLL(exon 5-8)–GPHN Δex14 showed some dimer formation but no clearly detectable higher molecular weight complex. The experiments were repeated at least 3 times with the same results.
Oligomerization of MLL-GPHN full and ex14. The 293T cells transiently expressing MLL(exon 5-8)–GPHN or its mutants were cross-linked in vivo and analyzed by Western blot after SDS-PAGE in reducing (R) or nonreducing conditions (NR) using anti-Flag antibody (B). The arrowheads indicate the cross-linked species. Cells expressing Flag-tagged TEL were used as a positive control for detecting oligomerization (A).
Oligomerization of MLL-GPHN full and ex14. The 293T cells transiently expressing MLL(exon 5-8)–GPHN or its mutants were cross-linked in vivo and analyzed by Western blot after SDS-PAGE in reducing (R) or nonreducing conditions (NR) using anti-Flag antibody (B). The arrowheads indicate the cross-linked species. Cells expressing Flag-tagged TEL were used as a positive control for detecting oligomerization (A).
GPHN itself has no transcriptional activation or repression activity
These data suggested that GPHN had both the capacity to convert MLL to a transforming protein and that oligomerization into trimers and tetramers was at least a correlate of this activity. Oligomerization of MLL might indeed be sufficient provided GPHN and, in particular, GPHN exon 14 provided no other functional attributes including transcriptional regulation were present. We therefore investigated the transcriptional properties of GPHN in transient transfection experiments. Since GPHN has no DNA binding activity, plasmids expressing the GAL4 DBD (aa's 1-147) fused to the entire GPHN involved in the MLL fusion protein or exon 14 of GPHN were generated and tested in comparison with the common MLL fusion partner ENL (aa's 431-559), which is known to have transcriptional activation property in this context.9 As shown in Figure 6B, GAL4(DBD)–ENL activated the transcription from a reporter through binding to GAL4 binding sites by nearly 8-fold. In contrast, GAL4(DBD)–GPHN had no effect. To exclude that GPHN had transcriptional repressor activity, the effects of GAL4(DBD)–GPHN on GAL4(RE)5-tkluc vector were compared with those of transcriptional repressors HDAC1 and NcoR. GAL4(DBD) fused HDAC1 and NcoR suppressed the reporter activity as expected (Figure 6C). In contrast, GAL4(DBD)–GPHN showed no effects on the herpes simplex virus (HSV) thymidine kinase (TK) promoter-driven reporter activity (Figure 6C).
Discussion
The fusion partners of MLL in acute leukemia range widely, including nuclear transcriptional factors and cytoplasmic proteins with variable functions.1,2 The question is therefore whether any functional attributes are shared by these partners. Previous reports showed that minimally required domains for cellular transformation identified in several translocation partners, including ENL, ELL, AF10, AFX, and FKHRL1, have transcriptional activation properties.9,10,12-14 The most plausible hypothesis drawn by these results is that transcriptional activation properties of the fusion partner might be a common function of many MLL fusion partners, particularly those that are themselves nuclear proteins, and that this activity is essential for leukemogenesis.15 This hypothesis is supported by experiments with an artificial MLL chimeric protein fused with the transactivating protein of HSV, VP16. MLL-VP16 also has transforming activity15 in vitro, although a smaller portion of VP16 that still had transcriptional activity was not sufficient for transformation.13 However persuasive, this explanation might not cover all reported fusion partner genes, as some, especially those encoding cytoplasmic proteins, are believed to have no transcriptional domain at all.
GPHN, a rare fusion partner on which we previously reported,18,19 belongs to this type of fusion partner. We used retroviral transduction/transplantation assays to evaluate the hematopoietic cell transformation capacity of MLL-GPHN fusion as reported in studies of other MLL fusions10,12-14,26,35-37 and show here that, despite a demonstrable lack of transcriptional activity, MLL-GPHN transforms murine hematopoietic progenitor cells. Strikingly, the 15 amino acids encoded by small exon 14 of GPHN are sufficient to transform murine hematopoietic cells in vitro in the context of MLL fusion, and cells transformed by this mutant (MLL-GPHN exon 14) are leukemic in vivo. The cells transformed by full-length MLL-GPHN or MLL-GPHN exon 14 had primitive stem cell–like phenotypes (CD34+, c-kit+, Sca-1+, lineage–) and, in vivo, gave rise to primitive myeloid and, less frequently, to lymphoid leukemias or leukemia with both lineage markers. This finding parallels that reported previously for MLL-GAS738 and indicates that MLL-GPHN, like MLL-GAS7, can target multilineage hematopoietic stem cells. These data indicate that a critical function for leukemogenesis is contained in the small exon 14 of GPHN.
A vital clue to possible mechanisms involved in oncogenic conversion of MLL by genes encoding cytoplasmic proteins was provided by studies with an artificial fusion, MLL–β-galactosidase (LacZ). Knock-in of the MLL-LacZ also induced leukemia, albeit with a relatively long latency.16 LacZ itself has not been reported to have any transcriptional activity so far, but one common characteristic of LacZ and the naturally occurring fusion partner GPHN is their oligomerization capacity. LacZ is known to assemble and exist as a tetramer in its enzymatic active form.17 As well as LacZ, MoeA with molybdenum cofactor biosynthesis activity, which locates at the C-terminal of GPHN, also has a dimer structure as its functional form.39,40 In addition to this dimer-formation capacity attributed to the MoeA domain, GPHN also contains another domain that has an oligomerization capacity. This oligomerization capacity of GPHN is essential for an assembly of the inhibitory receptors such as GABA (γ-aminobutyric acid) and glycine receptors under the synaptic membrane of neuronal cells.21 Although the domain responsible for oligomerization has not been formally identified, the most plausible site is considered to be the small microtubule (MAP2, tau) homology domain between 2 enzymatic domains (MoaB/MogA and MoeA) and which is encoded by exon 14.25 This exon is retained in the MLL-GPHN fusion and, as we show here, is both necessary and sufficient for transformation. Although many groups have undertaken extensive studies to identify functional domains of GPHN, this small exon has not been reported to have any other activity than the suspected oligomerization capacity. From the cross-linking experiments we performed, the 15 amino acids encoded by exon 14 of GPHN can oligomerize MLL into tetramers, as does full-length GPHN.
Secondary structure prediction analysis of the 15 residues (VQSRCSSKENILRAS) of GPHN encoded by exon 14 fused to MLL suggests that this domain of GPHN is mainly unstructured (D. Barford, personal communication to M.G., December 2003). Although a weak potency to form α-helix is indicated for residues ENILR, the short length of this helix (1.5 turns of helix) indicates that it is unlikely that this domain forms a coiled-coil dimerization motif. Exon 14 of GPHN has sequence homology to the microtubule-associated protein tau and MAP2, which promote tubulin polymerization.25 Analysis of the 31 amino acids of tau repeats is also consistent with a largely unstructured domain, with a weak propensity to form β-strand (D. Barford, personal communication to M.G., December 2003). Nevertheless, there are data indicating functionality of this small domain. Tau can oligomerize in normal41 and pathologic neurodegenerative diseases known as tauopathies.42 In the latter, tau self-aggregates to form pathologic structures in neuronal cells and the tubulin-binding domain (microtubule binding repeats) of tau is considered to be essential for this activity.24 Synthetic, single tubulin-binding repeats of tau tend to form dimers that are generally more stable than the monomers43 indicating protein-protein interaction in this small domain. In addition, mutant MAP2 with only one tubulin-binding repeat is functional, showing activity to bind and stabilize the tubulin complex as does the wild-type protein.44 Clearly, however, the structural basis for oligomer formation by GPHN, via the exon 14–encoded domain, as well as the similar activity of tau and MAP2 remains unknown. Finally in this regard, it is interesting to note that MLL-GPHN ex14 mutant with Flag tag on its C-terminal side could neither transform murine hematopoietic progenitors nor form oligomers (data not shown). This may indicate that some steric effects conferred by GPHN exon 14 may be essential for cellular transformation by MLL-GPHN.
While this manuscript was in preparation 2 papers were published45,46 that indicated that dimerization of MLL could transform and immortalize hematopoietic cells. These authors showed that 2 cytoplasmic fusion partners of MLL, AF1p, and GAS7 with coiled-coil domains, or a synthetic FK506 binding protein (FKBP), induce dimerization of MLL and that these domains were both necessary and sufficient for transformation. Additionally, evidence was presented for transformation, via dimerization, activating known targets of MLL fusion including HOX genes and HOX cofactor Meis1. On the other hand, MLL fusion proteins containing the synthetic dimerization domain only modestly altered the in vitro growth properties of myeloid progenitors, which could not be sustained indefinitely.45
Our data confirm these findings with reference to an additional fusion partner of MLL (GPHN), which we show has no intrinsic transcriptional properties, and, additionally, provide evidence that oligomerization of MLL may be more potent than dimerization in oncogenic conversion. MLL-GPHN Δex14 mutant, which lacks exon 14, still forms dimers, probably because it retains the MoeA domain with dimerization capacity. But this mutant was only very weakly transforming in serial replating assays compared with MLL-GPHN ex14 mutant and the cells could not be maintained long-term in vitro. This suggests that dimer formation may be less effective compared with oligomers of MLL in transforming cells. However, as dimer formation by MLL-GPHN Δex14 was relatively weak (Figure 5B), there is a possibility that dimerization, if strong enough, could be sufficient for transformation.
There remains unresolved questions about the precise mechanism by which oligomerization of the N-terminal half of MLL triggers leukemogenesis by, for example, transcriptional activation of HOX genes. Because GPHN (and other cytoplasmic partners) has no DNA binding activity, oligomerization of MLL after fusion with GPHN could cause some conformational changes in MLL influencing its binding to other transcriptional coregulators or more directly to its target genes. Martin et al46 demonstrate that dimerized MLL binds with increased efficiency to cytosineguanine dinucleotide (CpG) islands within the HOX a9 locus.
Another possibility is suggested by the recent reports addressing the function of normal full-length MLL protein. MLL is proteolytically cleaved to 2 parts (MLL N-terminal fragment MLLN and C-terminal fragment MLLC) and they must interact with each other to avoid degradation and confer stability.47,48 Both the cleavage site within MLL (located within highly conserved D/GVDD and D/GADD motifs) and the interaction domain of MLLN with MLLC fragment (FYRN domain) are located down-stream of the MLL breakpoint cluster region in which almost all of the breakpoints of MLL in the MLL fusion protein reside. Loss of interacting domains with MLLC fragment in the MLL fusion protein could result in instability in the form of a fusion gene that contains only MLLN. Thus fusion partners might provide the MLLN fragment with stability via oligomerization. In this regard, GPHN itself acts as stabilizer of neuronal inhibitory receptor complex at the synaptic membrane of neuronal cells by assembling the receptor via its oligomerization capacity.21
We conclude that the oligomerization of MLL protein may play a key functional role in oncogenic conversion. A similar organizational impact has been described for other leukemogenic fusion proteins including RARα, AML1, and for several fusion partners of NUP98, where fusion proteins contribute coiled-coil regions facilitating oligomerization.27,49,50 It is likely that this type of structural alteration changes the way the MLL protein interacts with the nuclear matrix, chromatin, and transcriptional coregulators. The MLL gene can also be converted to an oncogene in acute myeloid leukemia by partial internal duplications (exon 2-8).51 This alteration presumably mimics the structural impact of some chimeric fusions, and, in the light of these recent data, it would be of interest to determine if the protein product of MLL in such cases self-oligomerizes.
Prepublished online as Blood First Edition Paper, January 29, 2004; DOI 10.1182/blood-2003-11-3817.
Supported by a specialist program grant from the Leukaemia Research Fund (United Kingdom) and by the Kay Kendal Leukaemia Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lyn Healy, Yvonne Sunners, Deborah Knight, and Felicia Hunte for technical support; Arthur Zelent for comment on manuscript; Dave Robertson for confocal microscopy; and David Barford for structural analysis of GPHN sequences.