Abstract
We have studied the mechanisms by which human cytomegalovirus (HCMV) infection of monocyte-derived dendritic cells (moDCs) contribute to immune suppression. Unlike infection of immature moDCs, infection of mature moDCs is not lytic and results in minimally decreased surface major histocompatibility complex (MHC) and costimulatory molecule expression. The presence of a small percentage of CMV-infected mature moDCs, or the transfer of supernatant from infected moDCs depleted of infectious virions, is nevertheless sufficient to cause marked inhibition of immunostimulation by normal uninfected moDCs. Neither viral nor human interleukin 10 (IL-10) nor transforming growth factor-β-1 (TGF-β-1) could account for this inhibition. In contrast, we show that infected mature moDCs lose surface CD83 while maintaining intracellular protein expression. Soluble CD83 accumulates in the supernatants of CMV-infected mature moDCs, and CD83 immunodepletion removes the inhibitory effect of these supernatants on normal DC immunostimulation. We have thus discovered a new mechanism by which HCMV infection may establish a nonlytic reservoir in mature moDCs that inhibits DC-mediated T-cell responses. (Blood. 2004;103:4207-4215)
Introduction
Human cytomegalovirus (HCMV) is an opportunistic pathogen that establishes life-long latency after primary infection, despite the strong antiviral immunity developed by healthy hosts. This immunity controls viral replication, although latently infected healthy individuals still shed virus intermittently. HCMV reactivates and replicates under conditions of immune suppression and can result in substantial morbidity and mortality despite antiviral drug therapy.1,2 Conversely, acute HCMV infection can exert transient cellular immunosuppression in healthy persons.3-5 HCMV infection can also increase immunosuppression after transplantation, leading to serious secondary bacterial or fungal infections.6-8 Like other herpesviruses, HCMV has adapted to its host and has evolved multiple strategies to escape innate and acquired immunity.9
The effector T-cell arm has received more attention than the afferent arm of the immune response against CMV infection. While T cells are a key component of the antiviral immune response and T lymphopenia is an obvious risk factor for HCMV disease, we concentrated instead on HCMV infection of dendritic cells (DCs). DCs are critical initiators of cellular immunity against viral pathogens like HCMV. The sequelae of HCMV infection of DCs, however, could account for much of the additional immune suppression associated with reactivation in immunocompromised patients or with acute infection of healthy individuals.
The myeloid lineage is an important reservoir of HCMV. HCMV latently infects CD34+ hematopoietic progenitor cells (HPCs),10,11 and the virus principally targets monocytes in the peripheral blood. Cellular differentiation plays a key role in the activation of HCMV replication in the myeloid lineage. Indeed, differentiation of CD34+ HPCs into myeloid progeny, or monocytes into macrophages, allows full replication and production of new viral particles.10,12-14 Cells bearing some markers of macrophages and dendritic cells from healthy donors can also reactivate and release HCMV after allogeneic stimulation.15
Numerous viruses target DCs. Some viral infections induce DC maturation and stimulate the development of virus-specific T-cell responses.16,17 Other viral infections result in immune suppression by lysis of DCs, impairment of DC function without lysis, or release of infectious virus to bystander cells.18-21 Recent reports have indicated that certain HCMV strains can infect monocyte-derived dendritic cells (moDCs),22-25 Langerhans cells (LCs),26 and mouse splenic DCs.27
HCMV infection down-regulates class I and II major histocompatibility complex (MHC) molecules on moDCs and diminishes DC stimulation of T cells against recall24 and allogeneic antigens (Ags).23,25 Others have suggested that HCMV-infected DCs suppress immunity by deleting T cells via CD95-CD95L interactions.23 There is no consensus, however, as to the susceptibility of immature versus mature DCs to HCMV infection and little data about mechanisms of impaired DC function.23,24,28
In the present study, we analyzed the differential susceptibility of immature and mature moDCs to infection with endothelial cell- and fibroblast-adapted strains of HCMV. We demonstrate that while only immature moDCs undergo productive lytic infection, infection of mature moDCs results in a reduced ability to stimulate T cells in an allogeneic mixed leukocyte reaction (MLR) via a novel mechanism, the release of soluble CD83. This mechanism may contribute to viral escape through the release of soluble CD83 by infected dendritic cells in vivo.
Materials and methods
Media and sera
All moDC cultures used RPMI with 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and 1% penicillin/streptomycin (Media Preparation Core Facility, Memorial Sloan-Kettering Cancer Center [MSKCC], New York, NY) supplemented with 4 mM l-glutamine (Gibco BRL Life Technologies, Carlsbad, CA), 55 μM 2-mercaptoethanol (2ME; Gibco BRL Life Technologies), and single-donor normal human serum (NHS) from healthy prescreened CMV-seronegative donors. NHS was used at 1% for generation of moDCs or 10% for functional assays (eg, alloMLR). MRC5 fibroblasts were cultured in modified Eagle medium (MEM) with nonessential amino acids and 1% penicillin/streptomycin (MSKCC Media Prep Core Facility) and supplemented with 4 mM l-glutamine (Gibco BRL Life Technologies) and 2% fetal calf serum (FCS; Gemini BioProducts, Calabasas, CA). Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial basal medium 2 (EBM-2; CC 3156) with endothelial growth medium 2 (EGM-2) additives (CC 4176), both from Clonetics-BioWhittaker (division of Cambrex, East Rutherford, NJ).
Generation of monocyte-derived dendritic cells (moDCs)
Healthy donors provided peripheral blood samples after signing informed consent for MSKCC institutional review board (IRB)-approved protocols in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were separated over Ficoll-Paque Plus (endotoxin-free; Amersham Biosciences, Uppsala, Sweden). Immature moDCs were generated over 5 to 6 days from the tissue culture plastic adherent PBMCs in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF; 1000 IU/mL; Immunex, Seattle WA; now Berlex, Mountfield, NJ) and interleukin 4 (IL-4; 500 IU/mL; R&D Systems, Minneapolis, MN) in complete RPMI-1%NHS. Mature moDCs were obtained after an additional 2 days of culture in the presence of IL-1-β (400 IU/mL; R&D Systems), IL-6 (1000 IU/mL; R&D Systems), tumor necrosis factor-α (TNF-α; 1100 IU/mL; R&D Systems), and prostaglandin-E2 (PGE2; 5 nmol/mL; Calbiochem, EMD-Biosciences, San Diego CA).29 Byday 7 to 8 of culture, large forward-scatter (FSC) HLA-DR bright cells were nonadherent and more than 95% CD83+.
Generation of fibroblast- and endothelial cell-propagated HCMV
Two clinical HCMV isolates, Bob-U and Bob-B, were obtained, respectively, from the urine and blood of 2 distinct patients in the microbiology laboratory of Avicenne Hospital (Bobigny, France). Primary isolates were split to grow on human fibroblasts (MRC5; Diagnostic Hybrids, Athens, OH) and on HUVECs (Clonetics-BioWhittaker) as published.30 HCMV strains propagated on fibroblasts (Bob-U/F, Bob-B/F) or endothelial cells (Bob-U/E, Bob-B/E) were used after 20 passages.
Preparation of viral stocks and moDC infection
Viral stocks were prepared from supernatants of infected MRC5 (fibroblast-propagated clinical strains and AD169) or HUVECs (endothelial cell-propagated clinical strains) displaying more than 90% cytopathic effect. Supernatants were clarified (1000g for 30 minutes, 4°C) and concentrated 30-fold by ultracentrifugation at 80 000g for 90 minutes (L7 Ultracentrifuge; Beckman, Palo Alto, CA). Viral stocks were frozen at -80°C and thawed once before single use. Titers were assessed by a classic plaque assay31 on a thawed aliquot. Immature and mature moDCs from the same sample were infected in parallel with the same viral stocks at a multiplicity of infection (MOI) of 1 plaque-forming unit (PFU) per cell, unless otherwise specified. Mock-infected controls used UV-B-inactivated (70 000 J) virus at the same MOI, and uninfected controls were cells in medium only.
Antibodies and secondary reagents for staining
Mouse antihuman monoclonal antibodies (mAbs) directly conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or PE-cyanine-5 (Cy5) included anti-HLA-DR FITC (clone L243), anti-CD86 PE (clone IT2.2), anti-CD80 PE (clone L307.4), and anti-CD25 PE (clone 2A3) from BD Pharmingen (San Diego, CA); anti-MHC class I FITC (clone W6/32; Sigma, St Louis, MO); and anti-CD83 PE (clone HB15a) and anti-immunoglobulin-like transcript (ILT)-3-PE-Cy5 (clone ZB3.8) from Immunotech (Marseille, France). Mouse isotype controls included immunoglobulin G2a (IgG2a)-FITC (clone Dak-G05; Dako, Carpinteria, CA), IgG1-PE (clone Dak-GO1; Dako), and IgG1-PE-Cy5 (clone 2T8-2F5; Immunotech).
All reagents to detect HCMV antigens were unconjugated mouse mAbs: anti-immediate-early 1 (anti-IE1; clone E13; Argene, North Massapequa, NY), anti-unique long 44 antigen (anti-UL44; clone 131; Biodesign, Saco, ME), and anti-glycoprotein B (gpB; clone 1-M-12; Biodesign). Matched unconjugated isotypes included IgG1 (clone 111711.11; R&D Systems) and IgG2a (clone 20102.1; R&D Systems). Secondary reagents consisted of FITC-conjugated (Biosource, Camarillo, CA) or Texas-Red-conjugated (Jackson ImmunoResearch, West Grove, PA) goat antimouse Abs. For 2- and 3-color staining, anti-IE1 was biotinylated using the DAKO ARKit (Dako), and allophycocyanin (APC)- or FITC-conjugated streptavidin (BD Pharmingen) was added in a second step.
Staining of infected cells
The same procedure was validated and used for staining (1) cell suspensions for cytofluorography, (2) cytocentrifuged cells on glass slides (30 000 cells, 28g for 5 minutes, Cytospin 3; Shandon, Pittsburgh, PA), or (3) MRC5 monolayers in culture. All incubations were performed without ambient light. Live cells were stained at 4°C for cytofluorography. Cytospins and MRC5 monolayers were first fixed in 5% vol/vol formalin for 30 minutes and then permeabilized with 0.5% vol/vol nonidet P-40 (NP-40) solution for 30 minutes, after which cells were stained at room temperature. Cells were incubated with unconjugated antibodies against viral antigens for 30 minutes, followed by an additional 30 minutes of incubation with fluorochrome-conjugated Abs. Propidium iodide was sometimes used for nuclear counterstaining on cytospins.
For combined identification of moDC surface epitopes and HCMV, cells were first stained with fluorochrome-conjugated mAbs then fixed and permeabilized for IE1 staining. Biotinylated anti-IE1 Abs were incubated with the permeabilized cells for 30 minutes. After 2 washes, fluorochrome-conjugated streptavidin was added. For surface and intracellular CD83 staining, fluorochrome-conjugated anti-CD83 was added with the streptavidin conjugated to a different fluorochrome. For combined detection of IE1 and UL44, fixed and permeabilized cells were first stained for the UL44 antigen then for IE1 using biotinylated anti-IE1.
Micrographs were generated using a confocal laser scanning microscope (Zeiss LSM510; Zeiss, Thornwood, NY). For cytofluorography, 10 000 events were acquired by live gating on a FACS Calibur (Becton Dickinson, Mountain View, CA) and analyzed with CellQuest software. Mean fluorescent intensities (MFIs) were calculated for non-HCMV-exposed moDCs and for moDCs from HCMV-infected cultures gated specifically for IE1-positive (infected) and IE1-negative (bystander, uninfected) subpopulations, with subtraction of the MFI of the respective isotype control for each of these 3 groups. The MFI for each DC epitope evaluated on the IE1-positive and IE1-negative subpopulations from the HCMV-infected cultures was then compared with the MFIs of non-HCMV-exposed moDCs (100%) at day +1 and day +4 after infection.
Viral release and titers
To determine the amount of virus released into HCMV-exposed moDC cultures (moDCs 30 000/mL), supernatants were either spun (1000g, 10 minutes) or filtered (0.45 μm) to remove cells. An equal amount of supernatant was titered daily by a rapid culture assay, in which the supernatant was inoculated on MRC5 fibroblasts (24-well plate; Costar, Corning, NY) by centrifugation (1000g, 30 minutes), followed 24 hours later by fixation, permeabilization, and staining IE1. Titers were calculated as the number of IE1-positive MRC5 nuclei per mL of supernatant.
To determine actual spread of HCMV from infected moDCs to MRC5, moDCs were trypsinized (15 minutes, 37°C) to remove virions adherent to the cell surface and then washed with FCS to neutralize the trypsin. MRC5 monolayers in a 24-well plate were inoculated daily with 2500 moDCs per well. After 6 days of coculture, cells were fixed, permeabilized, and stained for IE1. Viral release and productive infection of the MRC5 fibroblasts were calculated as the number of viral foci in the MRC5 monolayer per 2500 moDCs.
Mixed leukocyte reaction (MLR)
The plastic nonadherent PBMC fraction from CMV-seronegative donors was further purified for T cells by nonadherence and elution from a nylon wool column (Polysciences, Warrington, PA). Varying numbers of moDCs (immature or mature, from HCMV-exposed or nonexposed cultures) were mixed with 105 single-donor allogeneic T cells at the ratios indicated. Cells were cultured in RPMI-10% normal human serum at 37°C in 5% humidified CO2. The stimulatory capacity of a particular moDC population was based on the resulting T-cell proliferation measured by the incorporation of 1 μCi (0.037 MBq) (3H)-thymidine (3HTdR) per well added during the last 18 hours of a 5- to 6-day culture. Cells were harvested using a Skatron harvester (Lier, Norway) and counted in a 1205-Betaplate counter (Perkin Elmer, Downers Grove, IL).
Supernatant transfer experiments to test inhibition of the allogeneic MLR
Supernatants from infected and noninfected mature moDC cultures were harvested between day +3 and day +4 after infection, clarified (1000g, 10 minutes), and frozen once at -80°C before thawing for single-use testing. Supernatants were exposed to UV to inactivate any remaining HCMV, and absence of infectious particles was confirmed by rapid culture assay on MRC5. MLRs with uninfected mature moDCs were cultured in medium containing 50% or 5% transferred supernatant together with 50% RPMI-20% normal human serum (final 10% NHS) or 95% RPMI-10%NHS, respectively.
Blocking experiments
Allogeneic T cells were opsonized for 1 hour at 37°C before mixing with uninfected or infected moDCs. For CD95 (Fas)/CD95-L (Fas-L) blockade, T cells were incubated with CD95 antagonist Ab (clone ZB4; 2.5 μg/mL; Immunotech), agonist Ab (clone CH11, 1 μg/mL), or both Abs successively. Controls included isotype-matched, nonreactive Abs (mouse IgG1 and IgM; R&D Systems in the same concentrations). These Abs were added again in the same amounts on day 2 and day 4 of the MLRs. For transforming growth factor-β-1 (TGF-β-1) and human or viral IL-10 blockade, T cells were first opsonized with a mixture of Abs blocking IL-10R-α (15 μg/mL; clone 37607.11; R&D Systems), IL-10R-β (50 μg/mL; clone 90220; R&D Systems), and TGF-β-1 (10 μg/mL; clone; R&D Systems) or a mixture of isotype-matched nonreactive Abs (mouse IgG1 and chicken IgY; R&D Systems). Thereafter, the alloMLRs were cultured and evaluated for 3HTdR incorporation as described above.
Enzyme-linked immunosorbent assay (ELISA) for soluble CD83
Supernatants from uninfected and infected mature moDC cultures were centrifuged to remove cell debris (20 000g, 15 minutes, 4°C). Soluble CD83 concentrations were determined in a sandwich ELISA as published32 using a mouse anti-CD83 Ab (clone HB15a; Immunotech) and a rabbit anti-CD83 Ab (RA83). Each sample was tested at 2 different dilutions. Each dilution was tested for the presence of soluble CD83 and for nonspecific background, both in triplicate. Standard curves for the estimation of soluble CD83 concentration were generated using serial dilutions of CD83-Ig. The lowest dilution of the standard was the threshold value of 75 pg/mL.
CD83 immunodepletion
Supernatants from noninfected and infected mature moDC cultures were centrifuged to remove cell debris (20 000g, 15 minutes, 4°C). Supernatants were then incubated with 3 μg/mL of anti-CD83 (clone HB15a; Immunotech) or matched isotype (mouse IgG2a; clone 20102.1; R&D systems) for 30 minutes at 4°C with gentle rotation. Protein A immobilized on agarose beads (ImmunoPure; Pierce, Rockford, IL) was added overnight (4°C with gentle rotation). Agarose beads were pelleted by centrifugation (20 000g, 15 minutes, 4°C), and immunodepleted supernatants were harvested. Mock-depleted supernatants were processed identically except for an initial opsonization with an isotype control mAb instead of anti-CD83. These supernatants were either used directly or frozen at -80°C before thawing for single use and tested as in the transfer experiments described above.
Statistics
We used a paired, 2-tailed Student t test to assess extent of CD83 immunodepletion and whether T-cell proliferation was significantly different when stimulated by moDCs in the presence of CD83- or mock-immunodepleted supernatants from HCMV-infected and control moDCs.
Results
Immature and mature moDCs have different susceptibilities to defined HCMV strains
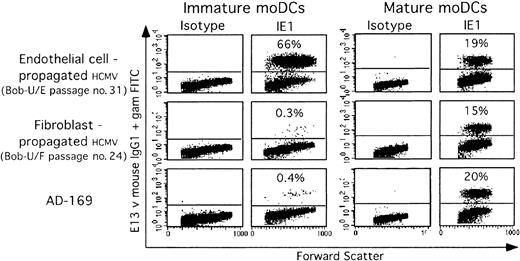
Cytofluorographic analysis detected infection 24 hours after moDC exposure to virus, based on immediate early 1 (IE1) viral antigen expression. Endothelial cell (EC)-strains at an MOI of 1 efficiently infected between 35% and 70% of immature moDCs (Figure 1). As previously reported,33 however, fibroblast (F)-strain infection of immature moDCs was very inefficient (Figure 1). In contrast, both F and EC strains infected mature moDCs similarly, albeit with a lower efficiency than EC-strain infection of immature moDCs (Figure 1). Infection rates varied between 15% and 30% at an MOI of 1, based on IE1 expression at day +1 after infection. Later analyses from days +2 through +4 after infection gave similar results.
Immature and mature moDCs have different susceptibilities to defined HCMV strains. Immature (left) and mature (right) moDCs from the same donor were infected in parallel with the same viral stocks (MOI-1) of different HCMV host cell-adapted strains: endothelial cell-propagated strains (Bob-U/E) or fibroblast-propagated strains (Bob-U/F and AD169). Twenty-four hours after infection, the immediate early-1 (IE1) viral antigen was detected in the nucleus of permeabilized, infected cells by using E13 Ab (1 experiment representative of 4).
Immature and mature moDCs have different susceptibilities to defined HCMV strains. Immature (left) and mature (right) moDCs from the same donor were infected in parallel with the same viral stocks (MOI-1) of different HCMV host cell-adapted strains: endothelial cell-propagated strains (Bob-U/E) or fibroblast-propagated strains (Bob-U/F and AD169). Twenty-four hours after infection, the immediate early-1 (IE1) viral antigen was detected in the nucleus of permeabilized, infected cells by using E13 Ab (1 experiment representative of 4).
HCMV infection decreases allostimulatory activity of both immature and mature moDCs
We tested moDC stimulatory activity in an alloMLR one day after infection with live or UV-inactivated HCMV. Both immature and mature moDCs infected with EC strains exhibited impaired allostimulatory activity compared with controls (Figure 2A-B). Stimulation by control immature moDCs was even more robust than usual because of the requisite manipulations used to mimic the handling in culture and transfer of the HCMV-infected moDCs.
HCMV infection of immature and mature moDCs impairs their allogeneic stimulatory activity. MoDCs were infected with EC- or F-propagated HCMV strains. Controls included medium alone without virus or UV-inactivated virus (mock infection). At day 1 after infection, moDCs were added as stimulator cells to allogeneic T cells from a CMV-seronegative donor. T cells (105 per triplicate U-bottomed well) were stimulated by different doses of moDCs in RPMI-10%NHS: (A) immature moDCs or (B) mature moDCs, both from the same donor and infected or not with EC strains, or (C) mature moDCs infected with F strains. Note y-axes use log2 scales to depict T-cell proliferation based on 3HTdR incorporation (counts per minute [cpm]) during the last 8 to 12 hours of a 5-day culture. (D) Percent inhibition of proliferation in the allogeneic MLR at a given DC/T-cell ratio (1:100) correlated with the rates of infection (% IE1-positive cells of the total DC exposed to HCMV) of the immature (♦) or mature (○) moDCs. Note that the lowest rates of infection were in the 3% to 5% range. r indicates coefficient of correlation.
HCMV infection of immature and mature moDCs impairs their allogeneic stimulatory activity. MoDCs were infected with EC- or F-propagated HCMV strains. Controls included medium alone without virus or UV-inactivated virus (mock infection). At day 1 after infection, moDCs were added as stimulator cells to allogeneic T cells from a CMV-seronegative donor. T cells (105 per triplicate U-bottomed well) were stimulated by different doses of moDCs in RPMI-10%NHS: (A) immature moDCs or (B) mature moDCs, both from the same donor and infected or not with EC strains, or (C) mature moDCs infected with F strains. Note y-axes use log2 scales to depict T-cell proliferation based on 3HTdR incorporation (counts per minute [cpm]) during the last 8 to 12 hours of a 5-day culture. (D) Percent inhibition of proliferation in the allogeneic MLR at a given DC/T-cell ratio (1:100) correlated with the rates of infection (% IE1-positive cells of the total DC exposed to HCMV) of the immature (♦) or mature (○) moDCs. Note that the lowest rates of infection were in the 3% to 5% range. r indicates coefficient of correlation.
At a DC/T-cell ratio of 1:100, allogeneic T-cell proliferation triggered by infected immature moDCs was still inhibited by 23% to 81% (n = 9 experiments), and that triggered by infected mature moDCs was inhibited by 22% to 65% (n = 7 experiments; Figure 2D). The rate of infection correlated with the degree of inhibition. We observed that even low rates of infection (eg, 3% to 5% IE1-positive cells) proved inhibitory (Figure 2D), however, suggesting bystander inhibition of noninfected moDCs in the same cultures. This bystander effect was particularly strong with mature moDCs (Figure 2D; see also Figures 6 and 7), which had lower rates of infection than immature moDCs yet were comparably inhibitory in the alloMLR.
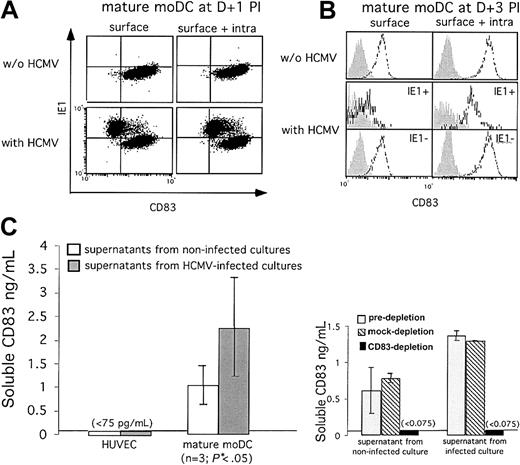
Mature moDCs infected with HCMV decrease membrane expression of CD83 and release increased amounts of soluble CD83 into the supernatant. CD83 rapidly disappears from the surface of IE1-positive mature moDCs but is still expressed intracellularly on (A) day +1 or (B) day +3 after infection (shaded histograms, isotype controls; open histograms, anti-CD83). Positive controls for surface CD83 expression comprise the non-HCMV-exposed moDCs as well as the bystander, uninfected and thus IE1-negative moDCs in the HCMV-exposed cultures. (C) ELISAs detected soluble CD83 in the supernatants of infected and uninfected mature moDC cultures (n = 3; mean ± SD), each assessed in triplicate. Infected mature moDC cultures released significantly more soluble CD83 than uninfected control cultures (*P < .05; Student t test). HCMV-infected and uninfected HUVECs, which never express CD83, provided the negative controls (left). Soluble CD83 could not be detected after CD83 immunodepletion (right).
Mature moDCs infected with HCMV decrease membrane expression of CD83 and release increased amounts of soluble CD83 into the supernatant. CD83 rapidly disappears from the surface of IE1-positive mature moDCs but is still expressed intracellularly on (A) day +1 or (B) day +3 after infection (shaded histograms, isotype controls; open histograms, anti-CD83). Positive controls for surface CD83 expression comprise the non-HCMV-exposed moDCs as well as the bystander, uninfected and thus IE1-negative moDCs in the HCMV-exposed cultures. (C) ELISAs detected soluble CD83 in the supernatants of infected and uninfected mature moDC cultures (n = 3; mean ± SD), each assessed in triplicate. Infected mature moDC cultures released significantly more soluble CD83 than uninfected control cultures (*P < .05; Student t test). HCMV-infected and uninfected HUVECs, which never express CD83, provided the negative controls (left). Soluble CD83 could not be detected after CD83 immunodepletion (right).
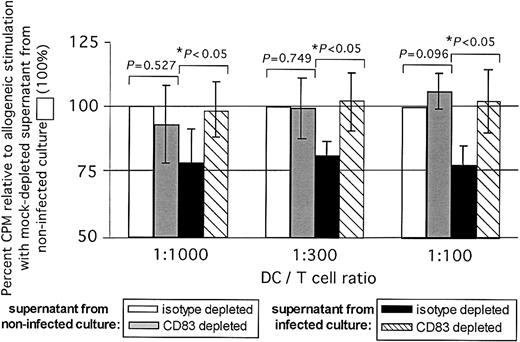
Immunodepletion of CD83 largely removes the inhibitory effect of supernatants from infected mature moDC cultures on DC-stimulated allogeneic T-cell responses in the MLR. Percent T-cell inhibition is shown against the y-axis, based on 3HTdR incorporation (cpm) relative to allogeneic stimulation with isotype (mock)-depleted supernatant from noninfected cultures, which by definition were at 100% (3 pooled independent experiments, mean ± SD). Supernatants were added at 50% vol/vol. T-cell proliferation stimulated by moDCs improved significantly when the supernatants added from infected cultures had been CD83 immunodepleted. *P values indicated on the figure were calculated using the Student t test comparing cpm values between mock-depleted and CD83-immunodepleted supernatants.
Immunodepletion of CD83 largely removes the inhibitory effect of supernatants from infected mature moDC cultures on DC-stimulated allogeneic T-cell responses in the MLR. Percent T-cell inhibition is shown against the y-axis, based on 3HTdR incorporation (cpm) relative to allogeneic stimulation with isotype (mock)-depleted supernatant from noninfected cultures, which by definition were at 100% (3 pooled independent experiments, mean ± SD). Supernatants were added at 50% vol/vol. T-cell proliferation stimulated by moDCs improved significantly when the supernatants added from infected cultures had been CD83 immunodepleted. *P values indicated on the figure were calculated using the Student t test comparing cpm values between mock-depleted and CD83-immunodepleted supernatants.
Serial assessments of proliferation during the 5-day MLR cultures did not reveal any earlier proliferation peaks (data not shown). Inhibition of moDC allostimulatory function required HCMV replication because mock infection with UV-inactivated EC strains exerted no effect (Figure 2A-B). Of note, infection of mature moDCs by AD169 and other F strains inhibited allostimulatory activity to a similar extent as infection with EC strains (Figure 2C).
HCMV infection is cytopathic and lytic in immature moDCs, whereas it enhances the survival of mature moDCs
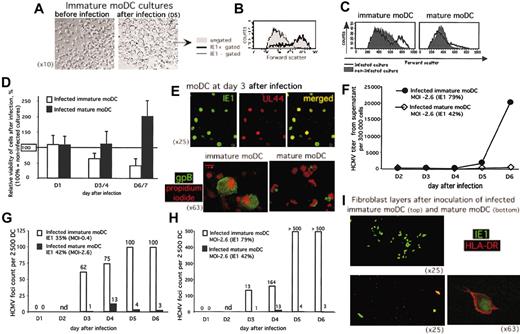
We investigated whether the decreased MLR stimulatory activity was simply the result of HCMV-induced lysis of moDCs. HCMV infection was cytopathic and lytic in immature moDCs, as already reported by others.22 Large refractile cells with smooth borders appeared in HCMV-infected cultures of immature (Figure 3A) but not mature moDCs (not shown). Flow cytometry provided objective confirmation of this cytomegalic effect of HCMV infection in the immature moDC cultures, because only cells that were infected and IE1-positive also exhibited large FSC (Figure 3B). Furthermore, the moDC cultures that were immature at the time of infection, but not the mature moDC cultures, included a substantially greater fraction of infected IE1-positive cells with cytomegalic changes from HCMV (large FSC; Figure 3C), despite roughly similar rates of infection (40% IE1-positive immature moDCs vs 30% IE1-positive mature moDCs).
HCMV infection of immature moDCs is cytopathic with lytic transmission of virus, while mature moDCs survive and do not productively transmit virus despite HCMV replication. (A) Inverse phase micrographs showing preinfection immature moDCs (left; original magnification, × 10) compared with enlarged, HCMV-infected immature moDCs (right; original magnification, × 10), further identified in panel B as the IE1-positive population (gated, thick solid line histogram, all large FSC) on the forward scatter histogram (gated IE1-negative cells plotted along the thin line histogram, all low FSC; and total population not gated for IE1 expression and showing a bimodal FSC distribution in the filled histogram). (C) Forward scatter histograms from uninfected cultures (filled histograms) and day-3 HCMV-infected cultures (thick lines, unfilled histograms) of immature moDCs (left) and mature moDCs (right) at similar rates of infection (40% IE1-positive immature moDCs vs 30% IE1-positive mature moDCs). (D) Viable cell counts were determined by trypan blue exclusion. The ratio of viable cells from infected cultures to viable cells from uninfected cultures × 100 was calculated to provide a percent relative viability. Each column represents the mean of 3 to 6 experiments ± SD; 100% viability was indexed according to the absolute number of viable cells in uninfected cultures on the assessment day indicated along the x-axis. Increased viability in the infected mature moDC cultures did not reflect expansion, as these moDCs were nondividing, but rather reflected enhanced viability relative to the uninfected cultures where there was some normal, unavoidable cell death in the absence of cytokines or other stimulus. Decreased viability of the infected immature moDCs reflected the lytic nature of the infection. Error bars indicate SD. (E) Expression of the immediate early-1 antigen IE1 (FITC) and early-late antigen UL44 (Texas-Red) (upper panels, all × 25 original magnification), and expression of the late antigen gpB (FITC) with PI (red fluorescence) (lower panels, both × 63 original magnification) with different patterns between immature and mature moDCs (see text). (F) HCMV titers from cell-free supernatants assessed by a rapid culture assay on MRC5 (1 experiment representative of 3). (G-H) HCMV cell-to-cell spread: 2500 EC-HCMV-infected moDCs were cultured on permissive fibroblast (MRC5) monolayers. Over 6 days, HCMV foci of infected fibroblasts were stained by anti-IE1-FITC and counted (nd indicates not done on day 2). Immature and mature moDCs were infected in parallel at different MOIs to achieve similar rates of infection (G) or in parallel at the same MOI, which resulted in different rates of infection (H) (1 experiment representative of 3 shown for each). (I) Confocal micrographs of fibroblast layers after coculture with day +5 HCMV-infected immature or mature moDCs. Infected cells were detected by green staining with anti-IE1-FITC, and anti-HLA-DR-PE identified moDCs as red cells.
HCMV infection of immature moDCs is cytopathic with lytic transmission of virus, while mature moDCs survive and do not productively transmit virus despite HCMV replication. (A) Inverse phase micrographs showing preinfection immature moDCs (left; original magnification, × 10) compared with enlarged, HCMV-infected immature moDCs (right; original magnification, × 10), further identified in panel B as the IE1-positive population (gated, thick solid line histogram, all large FSC) on the forward scatter histogram (gated IE1-negative cells plotted along the thin line histogram, all low FSC; and total population not gated for IE1 expression and showing a bimodal FSC distribution in the filled histogram). (C) Forward scatter histograms from uninfected cultures (filled histograms) and day-3 HCMV-infected cultures (thick lines, unfilled histograms) of immature moDCs (left) and mature moDCs (right) at similar rates of infection (40% IE1-positive immature moDCs vs 30% IE1-positive mature moDCs). (D) Viable cell counts were determined by trypan blue exclusion. The ratio of viable cells from infected cultures to viable cells from uninfected cultures × 100 was calculated to provide a percent relative viability. Each column represents the mean of 3 to 6 experiments ± SD; 100% viability was indexed according to the absolute number of viable cells in uninfected cultures on the assessment day indicated along the x-axis. Increased viability in the infected mature moDC cultures did not reflect expansion, as these moDCs were nondividing, but rather reflected enhanced viability relative to the uninfected cultures where there was some normal, unavoidable cell death in the absence of cytokines or other stimulus. Decreased viability of the infected immature moDCs reflected the lytic nature of the infection. Error bars indicate SD. (E) Expression of the immediate early-1 antigen IE1 (FITC) and early-late antigen UL44 (Texas-Red) (upper panels, all × 25 original magnification), and expression of the late antigen gpB (FITC) with PI (red fluorescence) (lower panels, both × 63 original magnification) with different patterns between immature and mature moDCs (see text). (F) HCMV titers from cell-free supernatants assessed by a rapid culture assay on MRC5 (1 experiment representative of 3). (G-H) HCMV cell-to-cell spread: 2500 EC-HCMV-infected moDCs were cultured on permissive fibroblast (MRC5) monolayers. Over 6 days, HCMV foci of infected fibroblasts were stained by anti-IE1-FITC and counted (nd indicates not done on day 2). Immature and mature moDCs were infected in parallel at different MOIs to achieve similar rates of infection (G) or in parallel at the same MOI, which resulted in different rates of infection (H) (1 experiment representative of 3 shown for each). (I) Confocal micrographs of fibroblast layers after coculture with day +5 HCMV-infected immature or mature moDCs. Infected cells were detected by green staining with anti-IE1-FITC, and anti-HLA-DR-PE identified moDCs as red cells.
Immature moDC survival was similar in infected and noninfected cultures at day +1 after infection, but this survival decreased by days +6 to +7 after infection to less than 50% of the viability in noninfected cultures (Figure 3D). On the other hand, HCMV infection of mature moDCs maintained or even enhanced viability (Figure 3D), although total cell counts never increased. This suggested that effects of HCMV infection other than cytomegalic changes, lysis, and loss of DC numbers accounted for the compromised immunostimulatory activity of mature moDCs. We therefore investigated HCMV infection beyond immediate early replication steps that could explain different mechanisms for the decreases in immunostimulatory function of HCMV-infected immature and mature moDCs.
HCMV fully completes replication in all moDCs, but only immature moDCs release infectious virus that productively infects other susceptible cells
Insofar as HCMV infection of mature moDCs is not lytic, infection might be restricted to the immediate early step and therefore be abortive. We assessed expression of the early-late antigen UL44 and the late antigen gpB by confocal microscopy in both immature and mature moDCs to explore later steps of HCMV infection. Most of the IE1-positive immature and mature moDCs expressed the early-late protein UL44 from days +2 through +5 after infection (Figure 3E top). Both also expressed the late antigen gpB, which appeared 2 to 3 days after infection. These results supported complete HCMV replication in both immature and mature moDCs and excluded the possibility of abortive infection in mature moDCs.
Expression of the late viral antigen displayed distinct patterns between immature and mature moDCs, however. At day +3 after infection, there was bright, diffuse cytoplasmic anti-gpB fluorescence in immature moDCs, while mature moDCs exhibited discrete anti-gpB fluorescence in cytoplasmic vesicular compartments (Figure 3E bottom). In addition, only immature gpB-positive moDCs were noticeably enlarged, consistent with the previously observed cytomegalic effect of HCMV infection.
To determine whether immature and/or mature moDCs could release infectious virus, HCMV-infected moDCs were harvested at day +1 after infection, washed twice to remove free virus, and seeded in equal numbers to 24-well plates for 5 to 6 days. Daily HCMV titers were determined from cell-free supernatants. Immature moDCs released large amounts of virus into the culture supernatant, while mature moDCs hardly released any HCMV (Figure 3F). Hence, only HCMV-infected immature, but not mature, moDCs transmitted infection to permissive fibroblasts, based on the number of IE1-positive foci over a 6-day coculture (Figure 3G-H). This was true whether we compared similar rates of infection from different MOIs (Figure 3G) or used similar MOIs yielding different rates of infection (Figure 3H) between immature and mature moDCs. Dual staining revealed the persistence of IE1-positive, HLA-DR-positive mature (but not immature) moDCs on the MRC5 fibroblast monolayers 13 days after original infection of the moDCs (Figure 3I), confirming that only mature moDCs survived the infection.
Immature moDCs were therefore permissive to the complete replication of HCMV, which induced cytomegaly, cell lysis, and the release of infectious virus. Despite complete replication of HCMV in mature moDCs, however, these moDCs did not release infectious virus, indicating a block in the maturation of the viral particles or a cellular compartment retaining the infectivity. Hence, lysis and productive release of new virus could not account for the loss of stimulatory activity by mature moDCs.
HCMV induces marked down-regulation of MHC and costimulatory molecule expression on IE1-positive immature moDCs but exerts little effect on HCMV-infected mature moDCs
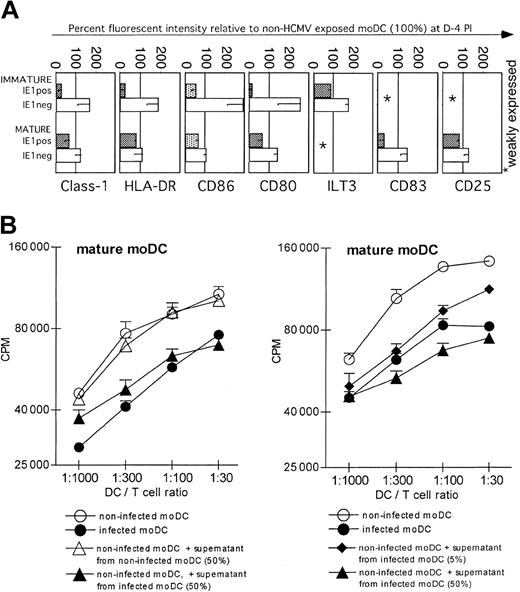
Consistent with previous reports24,28 we found that infected IE1-positive immature moDCs decreased expression of both CD80 and CD86, as well as class I and II MHC molecules, but maintained expression of ILT-3, for example, indicating that there was not a general shutdown of surface molecule synthesis due to HCMV infection (Figure 4A). These changes occurred by day +1 after infection, were maximal between days +3 and +5, and required viral replication because UV-inactivated HCMV did not exert the same effects (data not shown).
Mature moDCs are more resistant than immature moDCs to HCMV-induced down-regulation of surface MHC and costimulatory epitopes, and they secrete a transferable factor(s) that inhibits DC-stimulated T-cell responses. (A) Percent fluorescent intensity of HCMV-exposed relative to non-HCMV-exposed moDCs (100%). HCMV-infected, IE1-positive immature moDCs down-regulated class I and II MHC, CD86, and CD80 expression, whereas bystander, uninfected, IE1-negative cells from the same HCMV-exposed cultures up-regulated these epitopes (top). Unlike immature moDCs, however, HCMV exposure only slightly affected the phenotype of mature moDCs (bottom). The differences between IE1-positive and IE1-negative cells were much less in mature than in immature moDC cultures exposed to HCMV, with the exception that infected IE1-positive cells markedly down-regulated surface CD83 (bottom). (B) Virus-free supernatants (UV-B irradiated and no transmission to fibroblasts) from infected mature moDC cultures inhibit T-cell proliferation in the MLR in response to stimulation by uninfected mature moDCs (left; one experiment of 6 with similar results). The inhibitory effect of the supernatant was dose dependent (right). Each panel also includes infected moDCs as a positive control. Note that the y-axes use log2 scales to depict T-cell proliferation based on 3HTdR incorporation (cpm) during the last 8 to 12 hours of a 5-day alloMLR culture. Error bars indicate SD.
Mature moDCs are more resistant than immature moDCs to HCMV-induced down-regulation of surface MHC and costimulatory epitopes, and they secrete a transferable factor(s) that inhibits DC-stimulated T-cell responses. (A) Percent fluorescent intensity of HCMV-exposed relative to non-HCMV-exposed moDCs (100%). HCMV-infected, IE1-positive immature moDCs down-regulated class I and II MHC, CD86, and CD80 expression, whereas bystander, uninfected, IE1-negative cells from the same HCMV-exposed cultures up-regulated these epitopes (top). Unlike immature moDCs, however, HCMV exposure only slightly affected the phenotype of mature moDCs (bottom). The differences between IE1-positive and IE1-negative cells were much less in mature than in immature moDC cultures exposed to HCMV, with the exception that infected IE1-positive cells markedly down-regulated surface CD83 (bottom). (B) Virus-free supernatants (UV-B irradiated and no transmission to fibroblasts) from infected mature moDC cultures inhibit T-cell proliferation in the MLR in response to stimulation by uninfected mature moDCs (left; one experiment of 6 with similar results). The inhibitory effect of the supernatant was dose dependent (right). Each panel also includes infected moDCs as a positive control. Note that the y-axes use log2 scales to depict T-cell proliferation based on 3HTdR incorporation (cpm) during the last 8 to 12 hours of a 5-day alloMLR culture. Error bars indicate SD.
Bystander uninfected IE1 cells from the same cultures, in contrast, up-regulated these epitopes (Figure 4A) as well as the activation and maturation markers CD25 and CD83 (although the percent change could not be depicted relative to control immature moDCs that did not express these markers at baseline). As previously shown,24 IE1-positive immature moDCs, but not bystander IE1 cells, proved resistant to maturation when inflammatory stimuli were added after infection (Figure S1; see the Supplemental Figure link at the top of the online article on the Blood website).
Unlike infection of immature moDCs, however, infection of mature moDCs had comparatively little effect on the expression of MHC and costimulatory or activation molecules. A notable exception was the decreased surface expression of CD83 on infected IE1-positive mature moDCs, whereas it was slightly up-regulated on IE1 mature DCs from the same cultures (Figure 4A).
These results identified several causes for the impaired function of IE1-positive immature moDCs but did not fully account for the impaired immunostimulatory activity of infected mature moDCs. Furthermore, they did not explain the bystander inhibition of MLR activity that we have observed at low rates of infection. We therefore investigated whether infected mature moDCs secreted a soluble inhibitor(s) of moDC function or T-cell proliferation.
HCMV-infected mature moDCs produce soluble factor(s) that inhibit the immunostimulatory activity of uninfected mature moDCs
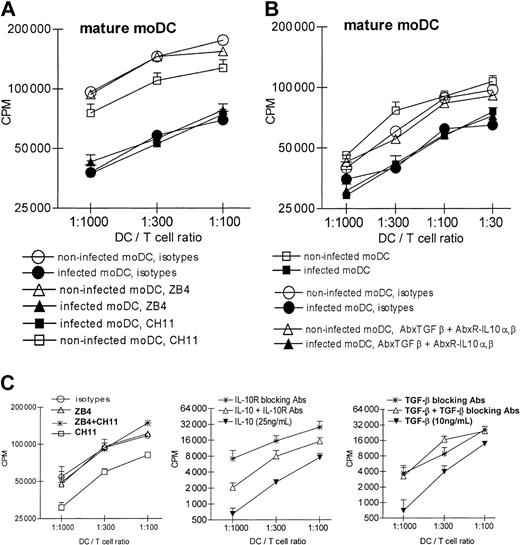
Frozen, once-thawed supernatants were UV irradiated and proven noninfectious by inoculation on fibroblasts. The supernatants from infected cultures of mature moDCs inhibited an alloMLR stimulated by uninfected moDCs in a dose-dependent manner (Figure 4B). Mature moDCs infected by HCMV therefore released a soluble factor(s) that suppressed moDC function and/or perhaps even T-cell proliferation directly. Supernatants from uninfected control cultures did not mediate the same inhibition. We excluded 3 potential direct inhibitory effects on T-cell proliferation by HCMV-infected moDCs. First, blockade of the CD95-CD95L pathway never restored the allostimulatory activity of HCMV-infected moDCs (Figure 5A), even though the same CD95L/FasL-blocking Ab ZB4 reversed MLR suppression by the CD95/Fas-activating Ab CH11 (Figure 5C left panel). Furthermore, blocking Abs to TGF-β-1 and/or IL-10R did not restore normal immunostimulatory activity of HCMV-infected DCs (Figure 5B), even though these Abs reversed the suppression induced by TGF-β-1 and IL-10 added to control alloMLRs (Figure 5C middle and right).
Blockade of CD95-CD95L interactions, TGF-β, or IL-10 does not restore the inhibition of T-cell stimulation by HCMV-infected mature moDCs. (A) Allogeneic T cells were preincubated with CD95 antagonist Ab (ZB4, 2.5 μg/mL), agonist Ab (CH11, 1 μg/mL), or both Abs successively. Controls included isotype-matched, nonreactive Abs in the same concentrations. Abs were added again during the MLR at days +2 and +4. Blockade of the CD95/CD95L interaction did not restore the immunostimulatory capacity of infected moDCs in the allogeneic MLR. (B) Allogeneic T cells were preincubated with a mixture of Abs blocking IL-10R-α (15 μg/mL), IL-10R-β (50 μg/mL), and TGF-β (10 μg/mL). Controls included a mixture of isotype-matched nonreactive Abs in the same concentrations or medium alone without Ab. Blocking IL-10 and TGF-β did not restore the immunostimulatory capacity of infected moDCs toward allogeneic T cells in the MLR. Note that the y-axes use log2 scales to depict T-cell proliferation based on 3HTdR incorporation (cpm) during the last 8 to 12 hours of a 5-day alloMLR culture. (C) Positive controls using noninfected moDCs confirmed the appropriate activity of the mAbs used in panels A and B. Error bars indicate SD.
Blockade of CD95-CD95L interactions, TGF-β, or IL-10 does not restore the inhibition of T-cell stimulation by HCMV-infected mature moDCs. (A) Allogeneic T cells were preincubated with CD95 antagonist Ab (ZB4, 2.5 μg/mL), agonist Ab (CH11, 1 μg/mL), or both Abs successively. Controls included isotype-matched, nonreactive Abs in the same concentrations. Abs were added again during the MLR at days +2 and +4. Blockade of the CD95/CD95L interaction did not restore the immunostimulatory capacity of infected moDCs in the allogeneic MLR. (B) Allogeneic T cells were preincubated with a mixture of Abs blocking IL-10R-α (15 μg/mL), IL-10R-β (50 μg/mL), and TGF-β (10 μg/mL). Controls included a mixture of isotype-matched nonreactive Abs in the same concentrations or medium alone without Ab. Blocking IL-10 and TGF-β did not restore the immunostimulatory capacity of infected moDCs toward allogeneic T cells in the MLR. Note that the y-axes use log2 scales to depict T-cell proliferation based on 3HTdR incorporation (cpm) during the last 8 to 12 hours of a 5-day alloMLR culture. (C) Positive controls using noninfected moDCs confirmed the appropriate activity of the mAbs used in panels A and B. Error bars indicate SD.
Mature moDCs infected with HCMV decrease membrane expression of CD83 and release increased amounts of soluble CD83 into the supernatant
A principal effect of HCMV infection was a marked decrease in CD83 at the cell surface of IE1-positive mature moDCs, which occurred very soon after infection and was already maximal at day +1 after infection (Figure 6A; see also Figure 4A). Permeabilized IE1-positive moDCs, however, still had detectable intracellular CD83 at day +1 and day +4 after infection (Figure 6A-B). This suggested that even though infected mature moDCs still expressed CD83, the molecule was somehow down-regulated or even shed from the cell surface. Using an antibody directed against the extracellular portion of the molecule in an ELISA,32 we detected a significant 2- to 3-fold increase in soluble CD83 in cell-free supernatants from HCMV-infected mature moDCs (Figure 6C left) compared with uninfected mature moDCs (P < .05; Student t test). Preincubation of supernatants with agarose bead-coupled anti-CD83 antibodies, but not isotype-matched antibodies, efficiently removed CD83 from the cell-free supernatants of HCMV-infected mature moDCs (Figure 6C right).
Immunodepletion of CD83 from the supernatant of HCMV-infected moDCs abrogates its inhibitory effect on DC stimulatory activity
Supernatants from infected versus uninfected mature moDC cultures were mock depleted or immunodepleted of CD83, requiring an overnight cold incubation with anti-CD83 and protein A as outlined in “Materials and methods.” The resulting supernatants were tested for their effects on the normal stimulatory function of uninfected mature moDCs in the alloMLR by addition at 50% vol/vol. Mock-depleted supernatants from HCMV-infected mature moDCs continued to inhibit the allostimulatory function of uninfected mature moDCs. The inhibitory effect was less than anticipated by the experiments in Figure 4B, consistent with some apparent loss of CD83 activity during the overnight cold incubation required to process the supernatants. Immunodepletion of soluble CD83 nevertheless restored allostimulatory DC function and normal T-cell proliferative responses (Figure 7; P < .05; 3 independent experiments), supporting the inhibitory biologic activity of CD83 released by HCMV-infected mature moDCs. CD83 immunodepletion of control supernatants from uninfected moDCs resulted in a much smaller increase in stimulatory function of moDCs and/or allogeneic T-cell proliferation (Figure 7; effect increases with dose of DCs), consistent with the small amounts of soluble CD83 lost from the cell surface even under normal conditions.
Discussion
We have investigated the effects of HCMV on DCs because of the formative role played by these cells in both innate and acquired cellular immunity. Our results illustrate that HCMV can weaken host immunity and promote its own survival by several mechanisms that vary with the maturation stage of moDCs.
HCMV infection of immature moDCs inhibits normal maturation, yet it induces the maturation of other cells that escape infection in the same cultures. These mature moDCs could potentially trigger strong immune responses, but we have demonstrated that mature moDCs are themselves susceptible to HCMV infection. Because HCMV infection of immature moDCs is lytic with release of substantial amounts of infectious virus, HCMV can use immature moDCs to amplify the viral load and spread to other susceptible cells like mature moDCs. HCMV infection in turn impairs mature moDC function through a bystander mechanism. CD83 released into the culture supernatant by infected mature moDCs inhibits the stimulatory activity of noninfected mature moDCs and/or T-cell proliferative responses. This new mechanism for viral escape may account for prolonged immune suppression, as infected mature moDCs that release inhibitory CD83 into the microenvironment have an enhanced survival themselves.
Both immature and mature moDCs infected by HCMV lose immunostimulatory function, as assessed in vitro by the alloMLR. By careful comparison of IE1-negative with IE1-positive moDCs within the same cultures, our results confirmed that HCMV infection induces substantial down-regulation of surface MHC and costimulatory molecules on infected immature moDCs. Our findings with immature moDCs were partially anticipated by similar results from other investigators,24,25 but we have emphasized important new findings regarding mature moDCs. Our studies are also the first in this area to have avoided the confounding effects of FCS in the DC cultures and to have used low MOIs usually of 1:1 but always less than 5:1.
What was not entirely anticipated by other studies using FCS24,25 was the degree to which uninfected immature moDCs from the same HCMV-exposed cultures could up-regulate MHC and costimulatory epitopes. In further contrast, HCMV infection has almost no effect in this regard on mature moDCs. HCMV is also not lytic in mature moDCs as it is in immature moDCs. This poses a particular puzzle regarding mature moDCs, which remain viable and maintain expression of critical MHC and accessory molecules yet have impaired T-cell stimulatory function. Recently published data have suggested an unidentified soluble factor as being responsible for inhibition of moDC stimulatory function, yet the positive control moDC activity in these studies was unusually weak, making further inhibition of questionable significance.25
In addressing this conundrum, we have detected a significant 2- to 3-fold excess of soluble CD83 in the supernatants of infected compared with uninfected mature moDCs. This correlates with a marked reduction in surface CD83 expression, despite the continued presence of CD83 protein within infected cells. The inhibition by direct transfer of supernatants from infected moDCs (Figure 4B) predicted a higher degree of inhibition by the mock-depleted versus CD83-immunodepleted supernatants evaluated in the experiments in Figure 7. We deduce that there is some loss of CD83 inhibitory activity in these supernatants, either because of the prolonged overnight cold incubation for immunodepletion or additional freeze-thaw cycle before addition to the alloMLRs, or both. Immunodepletion of the remaining soluble CD83 activity nevertheless completely abrogates the inhibitory effect transferred with cell-free supernatants. Another member of the family Herpesviridae, herpes simplex virus 1 (HSV-1), also infects mature moDCs with reduced CD83 expression and T-cell stimulatory capacity.18 Cell lysates after HSV-1 infection, however, had severely reduced CD83 protein levels indicating decreased synthesis18 and suggesting that HSV-1 and HCMV infection use distinct DC inhibitory mechanisms.
Intracellular CD83 is one of the best markers for commitment to the DC differentiation pathway, and its surface expression denotes stable maturation.34 Its functional role is not well established, however. During the process of maturation from immature moDCs, CD83 appears on the cell surface and can be recovered in soluble form from the culture supernatant.32 The recombinant extracellular domain of CD83 inhibits DC immunostimulatory capacity.35 Monocytes and a subset of activated CD8+ T cells36 or moDCs35 may express the receptor ligand for soluble CD83, which so far remains unidentified.
Our findings provide the first evidence for the release of a soluble form of CD83 by DCs upon viral infection. This contrasts with the severely reduced CD83 protein levels after HSV-1 infection.18,37 These data also indicate that soluble CD83 produced by mature moDCs has biologic activity, based on its inhibition of the DC-stimulated alloMLR and restoration of normal DC allostimulation and T-cell responsiveness when CD83 is removed. Much lower concentrations of released CD83 inhibited the alloMLR compared with the amount reported by other investigators who used the recombinant extracellular domain of CD83 made in Escherichia coli.35 A viral analog could not account for the biologic activity of soluble CD83 (sCD83) released by CMV-infected mature moDCs because sCD83 could not be detected in the supernatants of control CMV-infected HUVECs. The current results do not distinguish between an effect on moDCs or T cells or both, nor do these results exclude inhibition by additional unmeasured factor(s). The molecular basis for sCD83-mediated inhibition of DC-T-cell interactions still requires better definition and is under investigation.
Soluble CD83 could result from cellular secretion, shedding from surface membranes, or the presence of cellular and membrane debris as an artifact of culture in vitro. In support of either of the first 2 explanations is that CD83 immunodepletion removes most all of the inhibitory activity from the supernatants of infected cells. Against the possibility of inhibitory debris, cell viability was overall higher in infected than uninfected mature moDC cultures. CMV infection was also not lytic for mature moDCs, and all supernatants underwent centrifugation at 20 000g for 15 minutes before testing. We do not yet know whether alternative mRNA splicing or proteolytic cleavage accounts for the release of soluble CD83 from HCMV-infected mature moDCs.
Our results also could not impugn other factors like TGF-β or viral or human IL-10, consistent with studies in other settings of HCMV infection.28,38 Furthermore, we could not demonstrate a role for CD95/Fas in the inhibition of T-cell responses stimulated by HCMV-infected moDCs,23 as blockade of the CD95-CD95L pathway does not restore normal alloMLR responsiveness.
We have demonstrated the distinct susceptibilities of moDCs to HCMV infection, depending on their maturation state, and the attendant impairment of normal DC stimulation of T-cell proliferation due in large part to the release of soluble CD83 into the supernatant. This new mechanism of bystander immunosuppression involving soluble CD83 is not necessarily Ag-specific but may be important to the pathophysiology of HCMV infection. We did not address the consequences of DC infection, however, on either innate immunity or on acquired HCMV-specific immunity. Given the specific importance of CD4+ cells in maintaining CD8+ cytotoxic T lymphocyte (CTL) immunity against HCMV,39,40 the role of different types of DCs (eg, LCs, dermal dendritic cell-interstitial dendritic cells [DDC-IDCs], and moDCs) in stimulating CD4+ versus CD8+ responses against HCMV is of interest. Similarly, DCs are proving increasingly important in linking innate, natural killer (NK) cell-mediated immunity with acquired T-cell-mediated Ag-specific immunity in both mouse and human.41-45 Interferon producing cells (IPCs, pre-DC2) have also emerged as a critical line of defense against viruses46 and more specifically against CMV,47 substantiating the need for further investigation of these DCs in HCMV immunity.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-12-4350.
Supported by l'Association pour la Recherche sur le Cancer (B.S.); Charles A. Dana Foundation and the Mortimer Lacher Fellowship Fund (Memorial Sloan-Kettering Cancer Center; A.M.B.); LLS-6124-99 (J.W.Y.) from the Leukemia and Lymphoma Society; R01 CA 83070 (J.W.Y.), P01 CA 59350 (J.W.Y.), and P01 CA 23766 (J.W.Y.) from the National Cancer Institute, National Institutes of Health; and Mr William H. Goodwin and Mrs Alice Goodwin of the Commonwealth Cancer Foundation for Research from The Experimental Therapeutics Center, Memorial Sloan-Kettering Cancer Center (J.W.Y.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Dr Paul Deny (l'Hopital Avicenne, Universite de Paris XIII, Bobigny, France) for help and support in the development of new HCMV strains; Mr Jeffrey Stiles and Dr Timothy Kiehn (Clinical Microbiology Laboratory, Memorial Sloan-Kettering Cancer Center [MSKCC], New York, NY), as well as Dr Gabrielle Noffz and Mr Michael Basedow (MSKCC), for technical assistance; Drs David Munster and Masato Kato (Mater Medical Research Institute, South Brisbane, Queensland, Australia) for provision of CD83 reagents and critical guidance in the CD83 ELISA and immunodepletion experiments; and Drs Frederic Geissmann (Skirball Institute, New York University School of Medicine, New York, NY), Jianda Yuan, and Eric Pamer (both at Memorial Sloan-Kettering Cancer Center, New York, NY) for insightful discussions.

![Figure 2. HCMV infection of immature and mature moDCs impairs their allogeneic stimulatory activity. MoDCs were infected with EC- or F-propagated HCMV strains. Controls included medium alone without virus or UV-inactivated virus (mock infection). At day 1 after infection, moDCs were added as stimulator cells to allogeneic T cells from a CMV-seronegative donor. T cells (105 per triplicate U-bottomed well) were stimulated by different doses of moDCs in RPMI-10%NHS: (A) immature moDCs or (B) mature moDCs, both from the same donor and infected or not with EC strains, or (C) mature moDCs infected with F strains. Note y-axes use log2 scales to depict T-cell proliferation based on 3HTdR incorporation (counts per minute [cpm]) during the last 8 to 12 hours of a 5-day culture. (D) Percent inhibition of proliferation in the allogeneic MLR at a given DC/T-cell ratio (1:100) correlated with the rates of infection (% IE1-positive cells of the total DC exposed to HCMV) of the immature (♦) or mature (○) moDCs. Note that the lowest rates of infection were in the 3% to 5% range. r indicates coefficient of correlation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/11/10.1182_blood-2003-12-4350/6/m_zh80110462120002.jpeg?Expires=1766487555&Signature=dtYho6U8rMcZR7ZCYux7Rk0npuIai3zS6vVaoQufNrjpQv9TWtmv7mFkhMpHyrqjs1pliC0ozYkgO1hvJ0gsPbJn2EpnDGZiewBayrSVqtApB8P7HOreOCGeDwycwsWw5jGNbsgoaL~abuCh7Wj8b3Dl-rb9ZMdAuSudLPysXP4ZriyUh1LUdN5-70i-sSYpCxBqa2w4emctOpd-WDcobPT9jLq2lorbBOVTzjO-dzG8Zlt1EbaYVNYduOiQq0B658wq0KnWdZB8DXzPNmcYmyL9Iu6M28uIO7xN5jdczZVOpGkBNad-TXnS6RWfCQY7ckOH7nmobKRodY2Tp-U3QA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)