Abstract
The graft-versus-myeloma (GVM) effect of donor lymphocyte infusions (DLIs) is well established. We now report the outcome of DLI in 54 patients with relapsed myeloma following allogeneic transplantation. Twenty-eight patients (52%) responded, 19 patients (35%) with a partial response and 9 patients (17%) with a complete response. Progression-free and overall survival were 19 and 23 months, respectively. We found that acute and chronic graft-versus-host disease (GVHD) observed in 57% and 47% of patients, respectively, following DLI were the strongest predictors for response. This suggests that targets for GVHD and GVM are identical. In a subgroup analysis, deletion of chromosome 13, as determined by double-color fluorescence in situ hybridization (FISH), had no impact on outcome, indicating that these patients are candidates for early allogeneic transplantation followed by DLI, in case of insufficient response. (Blood. 2004;103:4362-4364)
Introduction
Donor lymphocyte infusions (DLIs) can induce remissions in patients with multiple myeloma who relapse after allogeneic stem cell transplantation (allo-SCT).1,2 Response rates between 30% and 50% have been reported.3,4 In a previous study with a limited number of patients, those with chemosensitive disease receiving a high T-cell dose seemed to have the best chance for response and prolonged survival.5 We recently updated the results of DLI in a larger group of patients and analyzed prognostic factors for response and survival.
Study design
Four transplantation centers in The Netherlands participated in the study of DLI administered for treatment of relapsed myeloma after allo-SCT. Approval was obtained from the participating centers' institutional review boards (University Medical Centers of Amsterdam, Rotterdam, Nijmegen, and Utrecht), and informed consent was provided by all patients according to the Declaration of Helsinki. Fifty-four patients, median age 52 years (range, 34-68 years), were included, 50 patients with a relapse following myeloablative partially T-cell-depleted allo-SCT6 and 4 patients following non-T-cell-depleted myeloablative allo-SCT. The first patients were conditioned with cyclophosphamide (120 mg/kg) and total body irradiation (TBI; 12 Gy) with lung shielding, and the latter patients were conditioned with low-dose TBI (2 Gy) and fludarabine (90 mg/m2).7 Patients received a total of 95 DLI courses (range, 1-7 courses) for a median of 20 months (range, 4-90 months) following transplantation. T-cell dose of DLI varied between 1 × 106 and 5 × 108 cells/kg. In the vast majority of patients, the starting dose was 1 × 107 T cells/kg. If after a minimum observation period of 3 months there was no response and no signs of graft-versus-host disease (GVHD), patients received a second course with 1 × 108 T cells/kg. A further dose escalation could be performed in the event of no response/GVHD following this second DLI. Forty patients received reinduction therapy before DLI that consisted of vincristine, adriamycin, dexamethasone (VAD; n = 32), dexamethasone as monotherapy (n = 7), or melphalan (70 mg/m2) as monotherapy. Responses to reinduction therapy and to DLI were assessed according to the criteria of the European Group for Blood and Marrow Transplantation (EBMT).8 A partial response (PR) was defined as decrease in myeloma proteins of at least 50% and improvement in myeloma-related signs/symptoms; a complete response (CR) was defined as absence of myeloma proteins as determined (at least twice, 3 weeks apart) by immunofixation of serum and urine and less than 5% plasma cells in the marrow. An ongoing response in patients already in PR was defined as additional decrease in myeloma proteins of at least 50% or achievement of CR.
Results and discussion
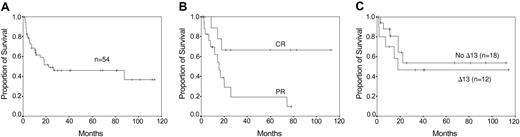
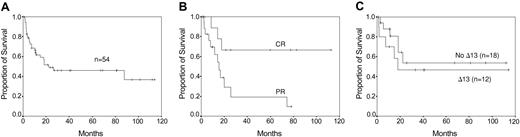
Eighteen of 40 patients treated with reinduction therapy achieved a PR: 14 of 32 patients after VAD, 3 of 7 patients after monotherapy with dexamethasone, and 1 of 1 patient after melphalan intravenously. Eleven of the 18 responding patients showed an ongoing response after DLI, including 7 patients with an additional 50% or higher reduction of tumor load and 4 patients who achieved a CR. Eight of 22 patients refractory to chemotherapy achieved a PR after DLI. Nine of 14 patients who did not receive reinduction treatment responded, including 4 patients with a PR and 5 patients with a CR. Altogether, 28 patients (52%) responded to DLI, 19 (35%) patients with a partial response and 9 (17%) with a complete response. This high response rate may be due to the fact that patients initially received T-cell-depleted transplants. Seventeen patients responded after the first course and 11 patients after dose escalation. Thirteen patients relapsed from DLI. Four of 5 relapsed patients responded again to a new course of DLI. Progression-free survival was a median of 19 months (range, 3-116+ months) and overall survival was a median of 23 months (range, 2-118+ months) (Figure 1A-B). Acute GVHD8 occurred in 31 patients (57%): 6 patients (11%) with grade I, 14 patients (26%) with grade II, and 11 patients (20%) with grades III-IV. Eighty percent of patients with GVHD grades II-IV responded to DLI, including 20% with a CR, whereas 33% of patients with GVHD grades 0-I responded, including 12% with CR. Chronic GVHD9 occurred in 25 patients (47%), limited GVHD in 9 patients (17%), and extensive GVHD in 16 patients (30%). Seventy-three percent of patients with chronic GVHD responded to DLI, including 9% with CR, whereas 37% of patients without chronic GVHD responded to DLI, including 22% with CR. Three patients (5%) died from toxicity: 2 patients from GVHD and 1 patient from septicemia.
Survival curves. (A) Overall survival after DLI. (B) Progression-free survival of patients responding to DLI. (C) Overall survival by deletion of chromosome 13 (Δ13) as determined by FISH.
Survival curves. (A) Overall survival after DLI. (B) Progression-free survival of patients responding to DLI. (C) Overall survival by deletion of chromosome 13 (Δ13) as determined by FISH.
In previous studies with a limited number of patients, chemosensitive disease and T-cell dose were significantly associated with response to DLI.5 In this study, which included a larger number of patients, it appeared that only the occurrence of acute GVHD grades II-IV and chronic GVHD and, to a lesser extent, response to reinduction therapy had an impact on response (Table 1). This observation strongly suggests that like leukemia,10 the targets for the cytotoxic donor (T) cells are minor histocompatibility antigens (mHa's) expressed on both recipient normal and myeloma plasma cells. We recently reported that donor-derived cytotoxic T-cell clones isolated from the peripheral blood of a myeloma patient responding to nonmyeloablative allo-SCT recognized both autologous normal B and malignant myeloma cells but not donor-derived B cells.11 It cannot be excluded however that non-antigen-specific mechanisms in association with GVHD, such as cytokines12 or tumor-specific antigens, are involved as well in graft-versus-myeloma (GVM). GVM may occur without GVHD and recently it was shown that in patients achieving a complete response to DLI this was associated with high antibody responses to highly expressed myeloma-associated antigens.13 One may speculate that although GVHD and GVM are strongly associated, both reactions are mediated by different T-cell populations triggered simultaneously. Functional studies of such T-cell populations after DLI/allo-SCT are needed to define which target antigens are involved in GVM. Other factors including T-cell dose, time interval between transplantation and DLI, GVHD after previous transplantation, and chimerism had no impact on response (Table 1).
Our study confirms the potential of the GVM effect of DLI to induce responses. However remissions were not long-lasting, especially not in patients with a PR. Only 6 patients are in sustained CR more than 2 years following DLI. A better long-term outcome may be achieved when post-DLI (maintenance) treatment is introduced with immune-modulating drugs like interferon or thalidomide. Interestingly, the deletion of chromosome 13 did not influence response and outcome of DLI (Figure 1C). We could determine this retrospectively by double-color fluorescence in situ hybridization (FISH) in the stored diagnostic bone marrow samples in 29 patients.14 However this must be confirmed prospectively in more patients, including those with cytogenetic-determined deletion of chromosome 13.
In conclusion, DLI is an effective treatment for myeloma patients with relapsed disease at the cost of acute and chronic GVHD in a substantial number of patients. The strong association between GVHD and GVM suggests that in both phenomena the same effector cells and target antigens are involved. Encouraging is the observation that GVM may overcome the adverse effect of deletion of chromosome 13.15,16 This entails that high-risk patients may be candidates for early donor stem cell transplantation followed by DLI in case of insufficient response.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-11-3862.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.