Abstract
Migration of dendritic cells (DCs) into tissues and secondary lymphoid organs plays a crucial role in the initiation of innate and adaptive immunity. In this article, we show that cyclosporin A (CsA) impairs the migration of DCs both in vitro and in vivo. Exposure of DCs to clinical concentrations of CsA neither induces apoptosis nor alters development but does impair cytokine secretion, chemokine receptor expression, and migration. In vitro, CsA impairs the migration of mouse bone marrow–derived DCs toward macrophage inflammatory protein-3β (MIP-3β) and induces them to retain responsiveness to MIP-1α after lipopolysaccharide (LPS)–stimulated DC maturation, while in vivo administration of CsA inhibits the migration of DCs out of skin and into the secondary lymphoid organs. CsA impairs chemokine receptor and cyclooxygenase-2 (COX-2) expression normally triggered in LPS-stimulated DCs; administration of exogenous prostaglandin E2 (PGE2) reverses the effects of CsA on chemokine receptor expression and DC migration. Inhibition of nuclear factor–κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathway signaling by CsA may be responsible for the CsA-mediated effects on the regulation of chemokine receptor and cyclooxygenase-2 (COX-2) expression. Impairment of DC migration due to inhibition of PGE2 production and regulation of chemokine receptor expression may contribute, in part, to CsA-mediated immunosuppression.
Introduction
Cyclosporin A (CsA) is an efficient immunosuppressive agent of significant clinical importance, widely used to treat organ transplantation, allergic disorders, autoimmune diseases, and acute inflammation.1 CsA acts by binding to immunophilins, a family of proteins that have peptidyl-prolyl cis-trans-isomerase activity,2-4 which, upon complexing with CsA, bind and inhibit the calcium-dependent protein phosphatase calcineurin.5,6 The effects of CsA on T lymphocytes have been extensively investigated. CsA inhibits antigen-dependent signaling events and decreases the proliferation of T cells in response to mitogens via inhibition of the nuclear factor of activated T cells (NFATs) and nuclear factor–κB (NF-κB)2,7,8 and prevents physiologic cell death in thymocytes and T-cell hybridomas.9,10 It has been recently demonstrated that CsA functions bidirectionally in antigen-specific dendritic cell (DC)–T-cell interactions.11 Thus, CsA acts at different steps of the immune response, including DC–T-cell interaction during antigen presentation and T-cell activation. However, little is known about the direct effects of CsA on DCs. In vitro, exposure of monocyte-derived DCs (MoDCs) to CsA does not affect cell phenotype or differentiation, although, in the presence of transforming growth factor-β (TGF-β), CsA can inhibit CD80 (B7-1) expression and decrease the antigen presentation capacity of DCs.12-14 In vivo administration of CsA also appears largely unable to significantly alter DC phenotype or function.15 However, some reports suggest that CsA can inhibit differentiation of Langerhans cells in rat epidermis,16 reduce the allostimulatory capacity and cytokine production of human myeloid DCs,17 and affect tumor necrosis factor-α (TNF-α) and lipopolysaccharide (LPS)–stimulated DC maturation.18 Further studies are required to confirm the direct effects of CsA on DCs and reconcile these conflicts.
DCs develop from bone marrow precursors and then migrate via the bloodstream to almost every tissue, where they reside as immature DCs.19 During pathogen invasion, or after exposure to inflammatory mediators, DCs undergo phenotypic and functional maturation.20 Upon maturation, DCs in tissues (skin, nonlymphoid interstitial sites, and mucosal surfaces) migrate into afferent lymphatics and move to the T-cell areas of lymph nodes, where they encounter naive T cells and initiate adaptive immune responses.19 Because naive T cells do not, for the most part, enter peripheral tissues, their interaction with and activation by DCs are dependent on the migration of DCs into secondary lymphoid tissues.21 This is apparent in mice lacking secondary lymphoid tissues, which cannot initiate alloimmune responses to vascularized organ transplants.22 Chemokine receptors (CKRs) are important in regulating DC localization and homing.23,24 Immature DCs express CXCR1, CCR1, CCR2, and CCR5, which confer responsiveness to inflammatory chemokines, directing the migration of DCs toward inflammatory stimuli and peripheral tissues in general.25,26 Upon exposure to maturation-inducing stimuli, such as inflammatory cytokines and pathogen products, DCs down-regulate CCR1 and CCR5, up-regulate CCR7 expression, and acquire responsiveness to CCL19/MIP-3β and CCL21/secondary lymphoid tissue chemokine (CCL21/SLC), facilitating emigration of DCs out of epidermis and immigration into secondary lymphoid organs.27-29 Deficiencies or alterations in CKR expression have been shown to profoundly affect immunity.30-33
Herein, we analyze the direct effects of CsA on DCs, using clinically relevant concentrations of CsA (150 to 300 ng/mL, equivalent 0.125 to 0.25 μM, therapeutic trough blood level).11 Bearing in mind observations that pharmacologic concentrations of CsA as high as 200 μM (250 μg/mL) can induce cell death in a variety of cell types,34-36 we first analyzed whether CsA induced apoptosis of bone marrow–derived murine DCs (BMDCs). We then examined whether CsA inhibits LPS-induced DC differentiation and phenotypic maturation and finally found that CsA can significantly alter the migratory capacity of DCs via regulation of CKR and cyclooxygenase-2 (COX-2) expression. Most important, we demonstrate that supplementation of CsA-treated DC cultures with exogenous prostaglandin E2 (PGE2) restores the migratory capacity of DCs. We suggest that CsA affects DC function at multiple steps during the immune response, including DC migration, and that PGE2, via control of CKR expression, is an important regulator of DC migration.
Materials and methods
Mice and reagents
Male wild-type C57BL/6 and BALB/c mice, 6 to 8 weeks of age, were obtained from SIPPR-BK Experimental Animal Company (Shanghai, China). GFP+/+ C57BL/6-TgN (ACTbEGFP) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice were housed in a pathogen-free facility for all experiments. Chemokine receptor antibodies (CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, and CXCR4), polyclonal antibodies (Abs) against actin and nucleoporin p62 (Nup62), and monoclonal Abs against COX-2 and NF-κBp65 were from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–labeled Abs against murine Iab, CD40, CD86, CD80, and CD11c; isotype control Abs; and FITC-labeled Abs against human CKRs were obtained from BD PharMingen (San Diego, CA). Phospho-Abs against extracellular signal-regulated kinase p44/p42 (ERK1/2, Thr202/Tyr204), c-Jun N-terminal kinase/stress-associated protein kinase (JNK/SAPK, Thr183/Tyr185), p38 (Thr180/Tyr182), and I-κBα (Ser32) and corresponding Abs against nonphosphorylated signaling proteins were from Cell Signaling Technology (Beverly, MA). Enzyme-linked immunosorbent assay (ELISA) kits for murine TNF-α, interleukin-12p70 (IL-12p70) and IL-10, PGE2, and recombinant murine macrophage inflammatory protein-1α (MIP-1α) CCL3 and MIP-3β CCL19 were purchased from R&D Systems (Minneapolis, MN). CsA, LPS, and PGE2 were from Calbiochem (San Diego, CA).
Generation of DCs
Mouse bone marrow–derived DCs (BMDCs) were prepared from bone marrow by culture with 20 ng/mL recombinant granulocyte macrophage colony-stimulating factor (GM-CSF) and 10 ng/mL IL-4 (Genzyme, Cambridge, MA), as described previously.37 Human peripheral blood monocyte-derived DCs (MoDCs) were cultured as described.38 On day 5, CsA and LPS treatments were performed as indicated. For analysis of CsA effects on DC development, CsA (0.1 to 1 μg/mL, equivalent 0.08 to 0.8 μM) was added on day 3.
Apoptosis assay
After treatments with CsA, DCs were labeled with annexin V and propidium iodide (PI) provided by BD PharMingen, following manufacturer's instructions. Mitochondrial membrane potential was measured by labeling cells with 1 μM rhodamine 123 (rho123) at 37°C for 15 minutes (Molecular Probes, Eugene, OR). The production of reactive oxygen species (ROS) was analyzed by labeling cells with 10 μM dihydrorhodamine 123 (DHR123) for 15 minutes (Molecular Probes). Samples were examined by fluorescence-activated cell sorter (FACS) analysis, and the results were analyzed using CellQuest software (Becton Dickinson, San Jose, CA).
Phenotype analysis
For detection of Ia, CD80, CD86, CD40, and CKR expression on BMDCs, cells were labeled and analyzed by FACS as described previously.39
MLR assay
MLR (mixed lymphocyte reaction) assay was performed as described previously.37 T cells were splenic T cells isolated from spleen of BALB/c mice by filtration through nylon wool. To exclude the possibility of CsA associated with CsA-treated DCs having carryover effects on T cells, DCs were washed extensively in fresh medium at least 3 times followed by 12-hour culture without CsA. The concentration of remnant CsA in culture supernatant and DCs was less than 1 ng/mL, as determined by ELISA using mouse monoclonal anti-CsA antibody-embedded plates. [3H]thymidine (0.5 μCi [1.85 × 104 Bq] per well; Amersham, Les Ulis, France) was added for the final 16 hours of culture. Cells were harvested using glass fiber filters, and thymidine incorporation was assessed using a β-scintillation counter (Wallac 1409; Wallac, Turku, Finland).
In vitro chemoattraction assay
To evaluate the responsiveness of CsA-treated DCs to chemokines (macrophage inflammatory protein-1α [MIP-1α] and MIP-3β), an in vitro chemoattraction assay was performed in 24-well transwell chambers (3 μm; Costar, Corning, NY), as described.37 Different amounts of MIP-1α or MIP-3β diluted in RPMI 1640 containing 0.5% bovine serum albumin (BSA) were added to lower wells in a volume of 0.6 mL; 0.1 mL RPMI 1640 containing 2 × 106 DCs was added to the upper wells and chambers incubated for 4 hours at 37°C. Directed migration was expressed as the number of CD11c+ cells that had migrated to the lower chamber, as counted by FACS.
Reverse transmigration assay
The reverse transmigration assay was performed mainly as described.40 107B is a murine splenic matrix endothelial cell line derived from C57BL/6 mice, verified and propagated in our laboratory. In brief, reverse transmigration filters (5-μm pore; Costar) were prepared by growing monolayers of 107B cells on the filters for at least 5 days; then cells were stripped away by treatment with 20 mM NH4OH plus 0.5% Triton X-100 (Sigma, St Louis, MO) for 30 seconds to form extracellular matrix. 107B cells were then grown to confluence on the extracellular matrix. The filters were placed upside down and mounted in Boyden chambers (Costar). A total of 1 × 106 C57BL/6 murine DCs were resuspended in RPMI 1640 medium containing 0.5% BSA and seeded into the upper compartment, and the lower compartment was filled with RPMI 1640 medium containing 0.5% BSA. The chamber was incubated for 4 hours at 37°C. Directed migration was expressed as the number of CD11c+ cells that had migrated to the lower chamber, counted by FACS. Each experiment was performed in triplicate, at least 3 times.
Preparation of cell suspensions from tissues
To prepare skin cell suspensions, abdominal skin samples were cut into small pieces and floated dermal side down in 0.5% trypsin (Sigma) dissolved in phosphate-buffered saline (PBS), for 40 minutes at 37°C. Samples were then placed in complete medium (RPMI 1640 containing 10% fetal calf serum [FCS]) supplemented with 0.25% DNase I (Sigma) at room temperature. After 20 minutes, the sheets were disrupted by vigorous pipetting, and the resulting cell suspensions were passed through nylon meshes. For the preparation of lymph node and splenocyte suspensions, samples were cut into pieces, passed through nylon meshes, cultured briefly in PBS containing 0.5% trypsin, and finally suspended in complete RPMI 1640 containing 10% FCS.
In vitro skin organ culture
To determine whether CsA treatment disrupted DC emigration out of skin, we performed in vitro skin organ cultures, as described.41 C57BL/6 mice were intraperitoneally injected with 20 mg/kg CsA every day for a total of 7 days. Abdominal skin biopsies were then taken, trimmed to uniform thickness, and cut to approximately 1 cm2 in size. The skin pieces were placed dermal side down on the lower membrane of the inner chamber of a 6-well double-chamber tissue culture plate (Costar) and incubated at 37°C. For the CsA-treated group, culture medium was supplemented with 1000 ng/mL (equivalent 0.8 μM) CsA. Forty-eight hours later, cells that had emigrated out of skin samples were collected and labeled with anti-CD11c (FITC) and counted by FACS.
In vivo DC migration assays
To determine whether the migration of CsA-treated DCs to secondary lymphoid organs was impaired, we transplanted BALB/c skin sheets (10- to 15-mm pieces) to C57BL/6 mice. Two days after transplantation, in vitro–cultured C57BL/6 DCs, with or without CsA treatment (1000 ng/mL for 24 hours), were labeled with the red fluorescence marker PKH-26 (Sigma) as described37 and immediately injected into skin allografts (1 × 106 in 100 μL). Six hours after injection, mice were killed and ipsilateral inguinal lymph nodes and spleens harvested, embedded in optimal cutting temperature (OCT) compound (IEC, Needham Heights, MA), and frozen immediately at –80°C. Cryostat sections (6 μm) were placed on cover slides, fixed in 1% paraformaldehyde for 5 minutes, incubated with 0.4 μg/mL Hoechst (Calbiochem) for 5 minutes, and subsequently examined by fluorescence microscopy. Cell suspensions from lymph nodes and spleen were also prepared and PKH-26–positive cells contained in these secondary lymphoid organs counted by FACS. To exclude the influence of in vitro labeling on DC function, green DCs derived from GFP+/+ (green fluorescent protein) C57BL/6 mice were used instead of PKH-26–labeled DCs.
To model normal/physiological in vivo DC migration as accurately as possible, C57BL/6 mice were treated with CsA for 7 days as described above, and then FITC painted was onto the abdomen, as described previously.42 The mice were intravenously injected with LPS (5 mg/kg) via the tail vein 12 hours after the final injection. Skin, spleen, and draining lymph nodes were isolated 12 hours later, and cell suspensions were prepared. DCs in the cell suspensions were stained with PE-labeled CD11c, and FITC/PE double-positive cells detected by FACS.
Chemokine receptor expression assay
For reverse transcriptase–polymerase chain reaction (RT-PCR), single-strand cDNA was synthesized from 2 μg total RNA using avian myeloblastosis virus (AMV)–RT (Promega, Madison, WI). Primers used for chemokine receptors were as follows: 5′-GTGTTCATCATTGGAGTGGTG-3′ and 5′-GGTTGAACAGGTAGATGCTGGTC-3′ for CCR1; 5′-GTGGGCAACATGTTGGTCATTATAA-3′ and 5′-GCCTCTTCTTCTCATTCCTACAGCG-3′ for CCR2; 5′-TTGCAGGACTGGCAGCATT-3′ and 5′-CCATAACGAGGAGAGGAAGAGCTA-3′ for CCR3; 5′-CCAGGCTACAGAAACCCTGG-3′ and 5′-TGTGTGGAGCTTGTTAACGC-3′ for CCR4; 5′-CATCGATTATGGTATGTCAGCACC-3′ and 5′-CAGAATGGTAGTGTGAGCAGGAA-3′ for CCR5; 5′-ACTCTTTGTCCTCACCCTACCG-3′ and 5′-ATCCTGCAGCTCGTATTTCTTG-3′ for CCR6; 5′-CCAGGAAAAACGTGCTGGTG-3′ and 5′-GGCCAGGTTGAGCAGGTAGG-3′ for CCR7; and 5′-CGGCAATGGATTGGTGATCCTGGTC-3′ and 5′-GAGGGCCTTGCGCTTCTGGTGGCCC-3′ for CXCR4. Primers used for mouse COX-2 were 5′-GGGTTGCTGGGGGAAGAAATGTG-3′ and 5′-GGTGGCTGTTTTGGTAGGCTGTG-3′. Primers used for murine β-actin were 5′-GTGGGAATTCGTCAGAAGGACTCCTATGTG-3′ and 5′-GAAGTCTAGAGCAACATAGCACAGCTTCTC-3′. Serially diluted cDNA (1:5 serial dilutions) was amplified by PCR under the following conditions: 25 to 27 cycles (depending on the saturation dilutions) of 30 seconds at 94°C, 30 seconds at 55°C or 57°C (depending on the primer pair), and 30 seconds at 72°C, followed by 10 minutes of elongation at 72°C. Using CCR7 as a standard, we determined that the proper dilution for the cDNA was 1:10. PCR products were resolved on a 1.5% agarose gel containing ethidium bromide and analyzed using a Hamamatsu Digital Camera system (Meyer Instruments, Houston, TX). For the detection of CKR protein expression by DCs, Western blot assay was performed as described.43 Proteins were revealed using Supersignal West Femto Maximum Sensitivity substrate (Pierce, Rockford, IL), following the manufacturer's instructions. Human monocyte-derived DCs were also subjected to Western blot assay and FACS assay for chemokine receptor expression.
MAPK signaling assay and NF-κB nuclear translocation assay
For analysis of mitogen-activated protein kinase (MAPK) signaling pathways, phospho-antibodies against ERK1/2, JNK/SAPK, and p38 were used to detect the expression levels of these molecules in whole cell lysates by Western blot. To examine the effects of CsA on NF-κB nuclear translocation, cell lysates were subjected to Western blot to detect phospho–I-κBα, and nuclear proteins, extracted using NE-PERTM nuclear reagents (Pierce), were Western blotted to detect the presence of NF-κBp65 in the nucleus.
Statistical analysis
Results are given as means ± SE or means ± SD. Comparisons between 2 groups were done using Student t test analysis, while comparisons between multiple groups were done using Kruskal-Wallis tests. Statistical significance was determined as P values less than .05.
Results
Clinical concentrations of CsA do not induce DC apoptosis
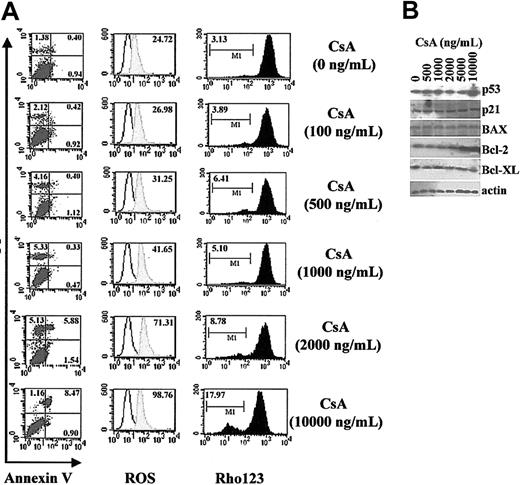
CsA has been demonstrated to induce apoptosis in glioma cells,34 endothelial cells,35 and fibroblasts36 either by regulating the production of reactive oxygen species (ROS) or by a p53-dependent pathway. However, there are also reports suggesting that CsA can protect cells from apoptosis due to its ability to block the mitochondrial permeability transition pore.44 To exclude the possibility that impairment of DC function mediated by CsA is due simply to a reduction of DC numbers via apoptosis, we first examined apoptotic sensitivity of BMDCs to CsA. On day 3 of DC culture, CsA at various dilutions (100 to 10 000 ng/mL, equivalent 0.08 to 0.8 μM) was added and cultures incubated for a further 72 hours. We found that low to moderate concentrations of CsA (up to 1000 ng/mL) could not induce apoptosis of DCs, as evidenced by annexin V and PI labeling (Figure 1A, left). The production of ROS (Figure 1A, middle) and mitochondrial potentials (Figure 1A, right) were not significantly altered, although the highest concentration of CsA (10 μg/mL) could induce, to some extent, an increase in ROS production and decrease in mitochondrial potential. CsA treatment did not induce significant up-regulation of proapoptotic factors p53, BAX, or p21 (Figure 1B). In contrast, we found that 1 μg/mL CsA up-regulated the expression of Bcl-2 although not Bcl-XL.
Clinical concentrations of CsA do not induce apoptosis of murine BMDCs. On day 3, DCs cultured in GM-CSF and IL-4 were treated with different concentrations of CsA, as indicated, for 72 hours. On day 6, DCs were harvested and analyzed. (A) Apoptosis assay of CsA-treated DCs. DCs treated with CsA were labeled with annexin V/PI (left; numbers indicate percentages of PI- or annexin V–positive cells), DHR123 (middle; labels indicate mean fluorescence intensity of shaded histogram), and rhodamine 123 (rho123; right; labels indicate percentage of R123low DCs) to detect early apoptosis, ROS production, and mitochondrial membrane potential, respectively. (B) Western blot analysis of apoptosis-associated molecules in DC lysates.
Clinical concentrations of CsA do not induce apoptosis of murine BMDCs. On day 3, DCs cultured in GM-CSF and IL-4 were treated with different concentrations of CsA, as indicated, for 72 hours. On day 6, DCs were harvested and analyzed. (A) Apoptosis assay of CsA-treated DCs. DCs treated with CsA were labeled with annexin V/PI (left; numbers indicate percentages of PI- or annexin V–positive cells), DHR123 (middle; labels indicate mean fluorescence intensity of shaded histogram), and rhodamine 123 (rho123; right; labels indicate percentage of R123low DCs) to detect early apoptosis, ROS production, and mitochondrial membrane potential, respectively. (B) Western blot analysis of apoptosis-associated molecules in DC lysates.
CsA does not affect differentiation but regulates the cytokine expression pattern of DCs
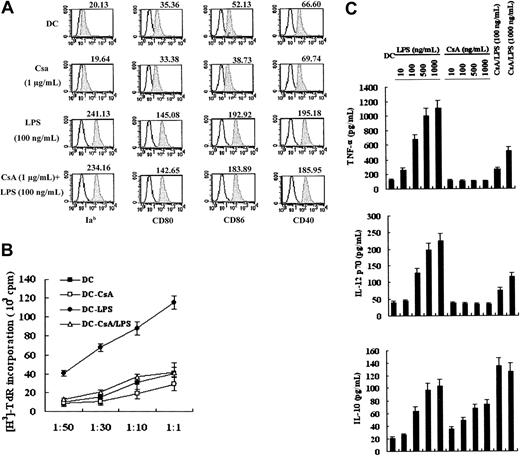
To examine whether CsA may impair the development of DCs derived from bone marrow, CsA (0.1 μg/mL to 1 μg/mL) was added on day 3 of DC culture, at the first culture medium change. We found that the number and ratio of CD11c+ cells generated by day 6 were not significantly altered by CsA inclusion (P > .05; data not shown). It has been demonstrated that CsA can inhibit the expression of costimulatory molecules on DCs.14-18 Surface expression of Iab, CD80, CD86, and CD40 on CsA-treated and -untreated DCs was examined by FACS analysis. CsA did not alter the phenotype of DCs (day 6) or the maturation of DCs (day 7) induced by LPS (Figure 2A). However, we did detect a significant decrease in allostimulatory capacity of CsA-treated LPS-matured DCs (Figure 2B; P < .05), which was not due to CsA “carryover,” because DCs were extensively washed with fresh medium and cultured for 12 hours without CsA prior to coculture of DCs with T cells. Cytokine secretion of LPS-stimulated CsA-treated DCs was measured by ELISA. CsA treatment inhibited production of Th1 cytokines TNF-α and IL-12p70 but increased production of the Th2 cytokine IL-10 (Figure 2C; P < .05).
Effects of CsA on murine BMDC differentiation, allostimulatory capacity, and cytokine production. (A) CsA does not affect DC differentiation. Day 3 DCs were stimulated with 1 μg/mL CsA for 72 hours. During the last 24 hours of CsA treatment, DCs were matured with 100 ng/mL LPS. DCs were then collected and analyzed for expression of major histocompatibility complex (MHC) (Ia) and costimulatory molecules (CD80, CD86, and CD40). Untreated day-7 DCs were used as a control (DC). Unfilled histograms depict isotype control, and shaded histograms represent surface expression level of developmental markers. (B) CsA inhibits the allostimulatory capacity of DCs. MLR was performed as described in “Materials and methods.” DC (day-7 DCs), DC-CsA (day-5 DCs treated with 1 μg/mL CsA alone for 48 hours), DC-LPS (day-6 DCs stimulated with 100 ng/mL LPS alone for 24 hours), and DC-CsA/LPS (day-5 DCs stimulated with both 1 μg/mL CsA and 100 ng/mL LPS) were used in this assay at various stimulator-responder (BALB/c splenic T cells) ratios, as indicated. (C) CsA inhibits TNF-α and IL-12p70 production but augments IL-10 production. Day 5 DCs were treated as indicated and were analyzed for cytokine production by ELISA. CsA concentration in CsA/LPS samples was 1 μg/mL. Results are expressed as means ± SEM (B-C).
Effects of CsA on murine BMDC differentiation, allostimulatory capacity, and cytokine production. (A) CsA does not affect DC differentiation. Day 3 DCs were stimulated with 1 μg/mL CsA for 72 hours. During the last 24 hours of CsA treatment, DCs were matured with 100 ng/mL LPS. DCs were then collected and analyzed for expression of major histocompatibility complex (MHC) (Ia) and costimulatory molecules (CD80, CD86, and CD40). Untreated day-7 DCs were used as a control (DC). Unfilled histograms depict isotype control, and shaded histograms represent surface expression level of developmental markers. (B) CsA inhibits the allostimulatory capacity of DCs. MLR was performed as described in “Materials and methods.” DC (day-7 DCs), DC-CsA (day-5 DCs treated with 1 μg/mL CsA alone for 48 hours), DC-LPS (day-6 DCs stimulated with 100 ng/mL LPS alone for 24 hours), and DC-CsA/LPS (day-5 DCs stimulated with both 1 μg/mL CsA and 100 ng/mL LPS) were used in this assay at various stimulator-responder (BALB/c splenic T cells) ratios, as indicated. (C) CsA inhibits TNF-α and IL-12p70 production but augments IL-10 production. Day 5 DCs were treated as indicated and were analyzed for cytokine production by ELISA. CsA concentration in CsA/LPS samples was 1 μg/mL. Results are expressed as means ± SEM (B-C).
CsA impairs DC migration in vitro and in vivo
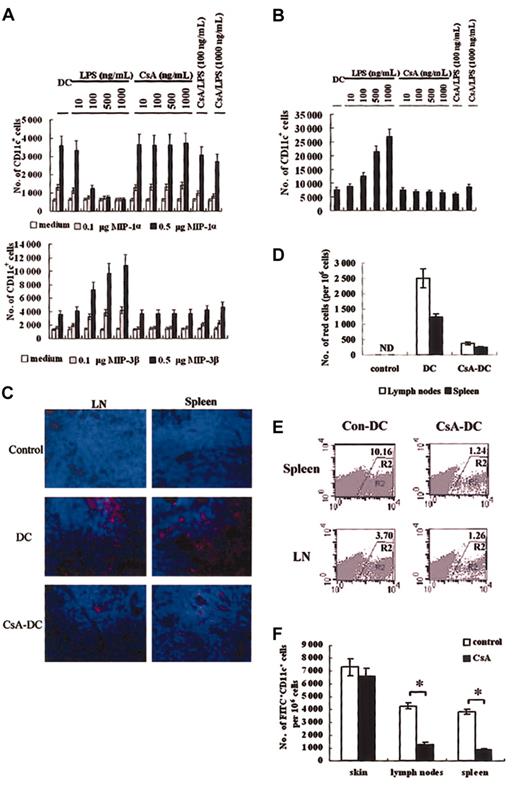
CsA can affect DC–T-cell interaction and inhibit T-cell function.11 It has been recently shown that CsA impairs the capacity of DCs to uptake antigens.45 In addition to the altered cytokine expression and decreased MLR capacity described above, we wondered if CsA may also act directly on DCs to regulate the immune response at other stages, including antigen uptake, antigen presentation, and migration of DCs. We found that CsA treatment alone could not significantly alter the migration pattern of DCs toward MIP-1α and MIP-3β (Figure 3A). After LPS-stimulated maturation, normal DCs lost responsiveness to MIP-1α and gained the capacity to respond to MIP-3β, as expected. However, CsA-treated LPS-matured DCs remained responsive to MIP-1α and only responded weakly to MIP-3β, suggesting that CsA treatment alters the migratory capacity of DCs. In the reverse transmigration assay, we found that DCs treated with CsA and matured with LPS could not migrate through the endothelial barrier whereas untreated LPS-matured DCs could (Figure 3B).
In vitro and in vivo DC migration assay. (A) In vitro chemoattraction assay of CsA- and LPS-treated murine DCs in response to MIP-1α (upper panel) or MIP-3β (lower panel). (B) Reverse transmigration assay of CsA-treated murine DCs. No chemokines were added to the lower chamber. In panels A-B, if not specifically indicated, the CsA concentration used was 1 μg/mL. (C-D) CsA treatment impairs PKH-26–labeled murine DC migration into spleen and lymph nodes (LN). (C) Immunofluorescence microscopy of PKH-26–positive cells in spleen and LNs. Results here are representative of sections derived from at least 3 samples. Red cells are PKH-26 labeled DCs, and blue cells are stained with Hoechst. Original magnification, × 400. (D) FACS enumeration of PKH-26–positive cells in spleen and LNs. ND indicates not detected. (E) FACS analysis of DCsGFP+/+ (R2 region) in spleen and LNs following injection into skin transplants. Labels indicate percentage of GFP+ cells in cell suspensions. (F) CsA does not affect numbers of FITC+CD11c+ DCs in skin but decreases the frequency of these cells in spleen and LNs. Mice treated with CsA (20 mg/kg) for 7 days were painted with FITC and then intravenously injected with LPS. Twelve hours later, FITC+CD11c+ cells in skin, spleen, and LN cell suspensions were counted using FACS. Results are expressed as means ± SD (A-B, D). Results in panel F represent 3 independent experiments. Asterisks in panel F indicate P < .05.
In vitro and in vivo DC migration assay. (A) In vitro chemoattraction assay of CsA- and LPS-treated murine DCs in response to MIP-1α (upper panel) or MIP-3β (lower panel). (B) Reverse transmigration assay of CsA-treated murine DCs. No chemokines were added to the lower chamber. In panels A-B, if not specifically indicated, the CsA concentration used was 1 μg/mL. (C-D) CsA treatment impairs PKH-26–labeled murine DC migration into spleen and lymph nodes (LN). (C) Immunofluorescence microscopy of PKH-26–positive cells in spleen and LNs. Results here are representative of sections derived from at least 3 samples. Red cells are PKH-26 labeled DCs, and blue cells are stained with Hoechst. Original magnification, × 400. (D) FACS enumeration of PKH-26–positive cells in spleen and LNs. ND indicates not detected. (E) FACS analysis of DCsGFP+/+ (R2 region) in spleen and LNs following injection into skin transplants. Labels indicate percentage of GFP+ cells in cell suspensions. (F) CsA does not affect numbers of FITC+CD11c+ DCs in skin but decreases the frequency of these cells in spleen and LNs. Mice treated with CsA (20 mg/kg) for 7 days were painted with FITC and then intravenously injected with LPS. Twelve hours later, FITC+CD11c+ cells in skin, spleen, and LN cell suspensions were counted using FACS. Results are expressed as means ± SD (A-B, D). Results in panel F represent 3 independent experiments. Asterisks in panel F indicate P < .05.
To further investigate the possibility that CsA treatment impaired either the capacity of DCs to emigrate out of skin epidermis or to immigrate into secondary lymphoid organs, we performed in vivo experiments to examine the migratory capacity of DCs in mice treated with CsA. Skin pieces from C57BL/6 mice treated with or without CsA were cultured in vitro for 48 hours; cells that had migrated out of skin tissues were then collected, labeled with CD11c, and CD11c+ cells detected by FACS. We found that DCs in CsA-treated mice showed a decreased ability to emigrate out of skin epidermis (1987 ± 86 versus 4036 ± 125 per 1 mm2 in untreated mice). However, there was no significant difference between the numbers of resident CD11c+ cells in skin of normal mice (8524 ± 264 per 106 cells) and CsA-treated mice (8289 ± 254 per 106 cells), as analyzed with FACS for CD11c+ cells in epidermal cell suspensions, which suggested that immigration of DCs into skin tissues was not affected. When DCs were treated with CsA, labeled with PKH-26, and subcutaneously injected into skin transplants of C57BL/6 mice derived from BALB/c mice (1 × 106 DCs per mouse), they showed dramatically reduced immigration into the spleen and lymph nodes as compared with untreated control DCs (Figure 3C). PKH-26–labeled cell numbers in spleen and lymph nodes were also enumerated by FACS; PKH-26–positive cells in secondary lymphoid organs were reduced in the CsA treatment group (Figure 3D). Cells were also isolated from skin transplants to determine levels of residual red-fluorescent DCs. We found that most of the injected CsA-treated DCs remained in skin pieces whereas most control-treated DCs migrated out (45.8% versus 14.6%, presented as: (remnant PKH-26 DCs in transplants/injected DCs) × 100%). When GFP-expressing DCs derived from C57BL/6GFP+/+ mice were used instead of PKH-26–labeled DCs, similar results were observed (Figure 3E). In the FITC-painting assay, following LPS mobilization, FITC-positive DC numbers in lymph nodes and spleen of CsA-treated mice were significantly lower than that in normal mice (Figure 3F). These results demonstrate that in vivo CsA treatment impairs the migration of DCs into secondary lymphoid organs.
CsA affects the migration of DCs by regulating CKR expression
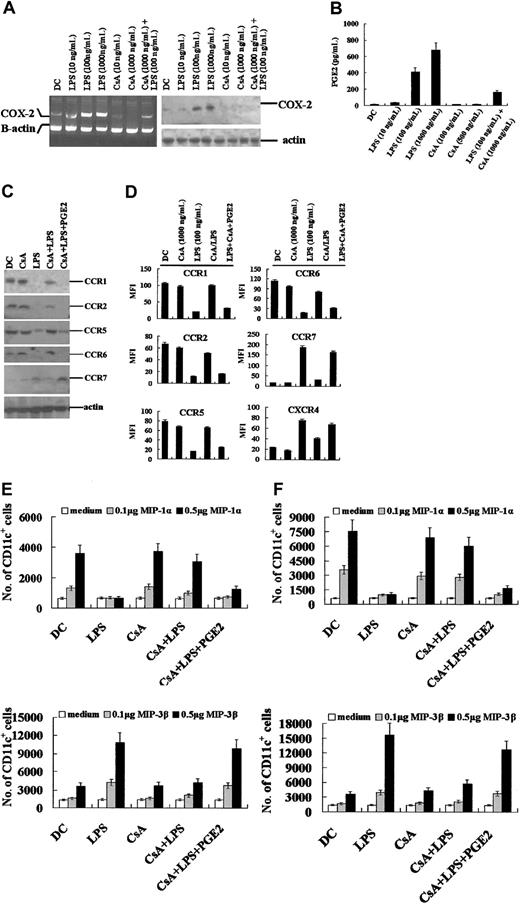
As demonstrated in the in vitro chemoattraction assay, CsA-treated DCs respond to MIP-1α but not to MIP-3β, even after LPS-induced maturation. We hypothesized that CsA treatment alters CKR expression, which in turn impairs DC migration. CCR1, CCR2, CCR5, and CCR6 expression remained high while CCR7 levels remained low in CsA-treated DCs after LPS stimulation, both at the mRNA and protein level (Figure 4A-B). However, LPS or CsA treatment did not significantly alter the expression of CCR3 and CCR4 on DCs. To further confirm these CsA-mediated effects on CKR expression, we examined CKR expression of human MoDCs by Western blot and FACS. Similar CsA-mediated alterations to CKR expression pattern were observed in human DCs (Figure 4C-D).
CsA treatments result in alterations to LPS-induced CKR expression. Murine BMDCs (day 5 DCs) were treated as indicated, harvested, and total RNA or total protein extracted. RT-PCR (A) and Western blot assay (B) were performed to examine CKR expression. (C-D) CKR expression in human MoDCs. DCs (day-6 DCs) were treated with CsA (1 μg/mL), LPS (100 ng/mL), or CsA (1 μg/mL) plus LPS (100 ng/mL) and then analyzed by Western blot (C) and FACS assays (D).
CsA treatments result in alterations to LPS-induced CKR expression. Murine BMDCs (day 5 DCs) were treated as indicated, harvested, and total RNA or total protein extracted. RT-PCR (A) and Western blot assay (B) were performed to examine CKR expression. (C-D) CKR expression in human MoDCs. DCs (day-6 DCs) were treated with CsA (1 μg/mL), LPS (100 ng/mL), or CsA (1 μg/mL) plus LPS (100 ng/mL) and then analyzed by Western blot (C) and FACS assays (D).
PGE2 rescues the CsA-induced migratory impairment of DCs
Cyclooxygenases (COX-1 and COX-2) are the major enzymes involved in PGE2 production.46,47 COX-2 is induced in response to multiple stimuli, including TNF-α, phorbol esters, hypoxia, IL-1α, and LPS. It has long been noted that CsA can inhibit the expression of COX-2 in several cell types, which in turn down-regulates the production of PGE2 triggered by various stimuli.46,47 Recently, PGE2 was found to cooperate with several stimulators to regulate the expression of CCR7 on DCs and their ensuing migratory capacity.48,49 We determined the effects of CsA on LPS-induced COX-2 expression and resultant PGE2 production. CsA treatment resulted in a decrease in LPS-stimulated COX-2 expression by DCs at both the mRNA and protein level (Figure 5A). PGE2 levels in culture supernatant were correspondingly inhibited by CsA treatment (Figure 5B). In the presence of exogenous PGE2 (1 μg/mL) and CsA (1 μg/mL, equivalent 0.8 μM), LPS-stimulated expression of CCR7, CCR1, CCR2, CCR5, and CCR6 was restored in both murine BMDCs (Figure 5C) and human MoDCs (Figure 5D), suggesting that CsA-induced CKR expression patterns resulted from inhibition of COX-2 expression and subsequent PGE2 production. Moreover, the migratory capacity of CsA-treated DCs was also restored, as demonstrated by in vitro chemoattraction assay (Figure 5E-F).
PGE2rescues the migratory pattern of CsA-treated DCs. (A) CsA inhibits LPS-induced COX-2 expression. Murine bone marrow–derived DCs were treated as indicated and COX-2 expression assayed by RT-PCR and Western blot assays. CsA/LPS: Day 5 DCs were treated with CsA (1 μg/mL) for 12 hours, and then LPS (100 ng/mL) was added for the final 12 hours. (B) CsA inhibits PGE2 production of LPS-stimulated murine DCs. (C) Western blot assay of CKR expression by murine BMDCs following supplementation with PGE2 (1 μg/mL). (D) FACS analysis of CKR expression on PGE2-supplemented human MoDCs. Results presented as mean fluorescence intensity (MFI) ± SE. (E-F) PGE2 restores responsiveness of CsA-treated murine BMDCs (E) and human MoDCs (F) to MIP-1α or MIP-3β. In panels C-F, day 5 DCs were treated by CsA (1 μg/mL) for 48 hours and stimulated by LPS (100 ng/mL) in the last 24 hours. Results in panels B and D are expressed as means ± SEM; results in panels E-F are expressed as means ± SD.
PGE2rescues the migratory pattern of CsA-treated DCs. (A) CsA inhibits LPS-induced COX-2 expression. Murine bone marrow–derived DCs were treated as indicated and COX-2 expression assayed by RT-PCR and Western blot assays. CsA/LPS: Day 5 DCs were treated with CsA (1 μg/mL) for 12 hours, and then LPS (100 ng/mL) was added for the final 12 hours. (B) CsA inhibits PGE2 production of LPS-stimulated murine DCs. (C) Western blot assay of CKR expression by murine BMDCs following supplementation with PGE2 (1 μg/mL). (D) FACS analysis of CKR expression on PGE2-supplemented human MoDCs. Results presented as mean fluorescence intensity (MFI) ± SE. (E-F) PGE2 restores responsiveness of CsA-treated murine BMDCs (E) and human MoDCs (F) to MIP-1α or MIP-3β. In panels C-F, day 5 DCs were treated by CsA (1 μg/mL) for 48 hours and stimulated by LPS (100 ng/mL) in the last 24 hours. Results in panels B and D are expressed as means ± SEM; results in panels E-F are expressed as means ± SD.
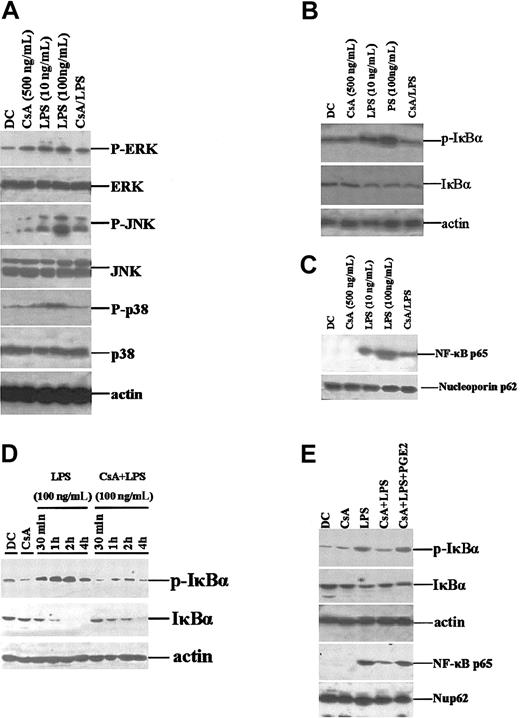
CsA inhibits the activation of MAPK and NF-κB
CsA functions by binding to and inhibiting the calcium-dependent protein phosphatase calcineurin, which then inhibits the transcription factors NFAT and NF-κB, in turn resulting in inhibition of gene expression, including that of cytokine genes.2-6 During DC differentiation, maturation, and migration, MAPK signaling pathways and NF-κB transcription play important roles.50,51 Recent studies have suggested that these 2 pathways are involved in the regulation of CKR expression.52,53 We examined whether these signaling pathways are involved in the CsA-mediated impairment of CKR expression. Upon LPS stimulation of DCs, the ERK, JNK, and p38 pathways were rapidly activated, whereas CsA-pretreated DCs showed decreased activation, evidenced by alterations in the levels of phosphorylated ERK, JNK, and p38 (Figure 6A). Under normal conditions, most NF-κB subunits are sequestered in the cytoplasm by I-κBα. Following I-κBα phosphorylation, NF-κB subunits translocate into the nucleus where they regulate expression of various genes. We found that phospho–I-κBα levels in total cell lysates of LPS-stimulated CsA-treated DCs were much lower than those in control DCs (Figure 6B). Nuclear NF-κB p65 subunit levels showed corresponding changes (Figure 6C). It was surprising to find that LPS stimulation for 30 minutes did not significantly decrease the total level of I-κBα. (Figure 6B). Thus, we observed the dynamics of I-κBα over a 4-hour LPS stimulation. We found that CsA could partially inhibit LPS-induced activation and degradation of I-κBα (Figure 6D) and that this effect could be partially reversed by exogenously supplemented PGE2 (Figure 6E). Therefore, CsA-mediated alterations in CKR expression in DCs may be partially due to inhibition of the MAPK and NF-κB signaling pathways.
CsA inhibits ERK, JNK, and p38 activation and NF-κB nuclear translocation. (A) CsA inhibits MAPK activation. Day 5 murine BMDCs were treated with CsA (1 μg/mL) for 12 hours or stimulated with LPS (100 ng/mL) for 30 minutes, at the indicated concentrations. Total cell lysates were prepared and analyzed for phospho-ERK1/2 (p-ERK), phospho-JNK (p-JNK), and phospho-p38 (p-p38) expression. Unphosphorylated proteins were also detected using specific antibodies. (B) CsA inhibits LPS-induced I-κBα activation. BMDCs were treated as in panel A. (C) CsA inhibits LPS-induced NF-κB nuclear translocation. Nuclear proteins were extracted from cells, treated as above, and Western blotted for NF-κBp65. (D) CsA inhibits the activation and degradation of I-κBα induced by pulsing with LPS. BMDCs were stimulated with LPS/CsA as indicated. (E) PGE2 restores the activation of I-κBα and NF-κBp65. BMDCs were treated as in panel A.
CsA inhibits ERK, JNK, and p38 activation and NF-κB nuclear translocation. (A) CsA inhibits MAPK activation. Day 5 murine BMDCs were treated with CsA (1 μg/mL) for 12 hours or stimulated with LPS (100 ng/mL) for 30 minutes, at the indicated concentrations. Total cell lysates were prepared and analyzed for phospho-ERK1/2 (p-ERK), phospho-JNK (p-JNK), and phospho-p38 (p-p38) expression. Unphosphorylated proteins were also detected using specific antibodies. (B) CsA inhibits LPS-induced I-κBα activation. BMDCs were treated as in panel A. (C) CsA inhibits LPS-induced NF-κB nuclear translocation. Nuclear proteins were extracted from cells, treated as above, and Western blotted for NF-κBp65. (D) CsA inhibits the activation and degradation of I-κBα induced by pulsing with LPS. BMDCs were stimulated with LPS/CsA as indicated. (E) PGE2 restores the activation of I-κBα and NF-κBp65. BMDCs were treated as in panel A.
Discussion
In vitro experiments have suggested that CsA can induce apoptosis in a variety of cell types via induction of ROS production or p53-dependent apoptosis.34-36 However, in our experiments, we could not detect either apoptosis, mitochondrial membrane potential decrease, or ROS increase upon DC exposure to clinical concentrations of CsA (100 to 300 ng/mL, equivalent 0.08 to 0.25 μM). Correspondingly, there was no detectable increase in apoptosis-inducing molecules or decrease in antiapoptotic factors in CsA-treated DCs. This observation is consistent with reports that CsA at moderate concentrations (0.5 to 2.5 μM, equivalent 600 to 3000 ng/mL) cannot induce apoptosis in endothelial cells,42 although oxidative stress can be observed, along with up-regulation of Bcl-2. Given that the apoptosis-inducing concentrations used of CsA were 200 μM (250 μg/mL), at least 100-fold stronger than typical clinical CsA concentrations, and that CsA-mediated apoptosis-inducing effects varied between cell types, it was not surprising for us to find that clinical concentrations of CsA were unable to induce apoptosis of DCs.
It has been reported that CsA negatively regulates the endocytic activity of immature CD11c+ DCs45 and that CsA can efficiently and uniformly block all changes resulting from intercellular signaling in both DC–T-cell and T-cell–DC directions.11 There are discrepancies between reports on the effects of CsA on expression of costimulatory molecules; however, when taken together with our study, it seems likely that CsA does not affect the differentiation and maturation of DCs in response to LPS. Consistent with reports by others, CsA inhibited production of IL-12p70 and TNF-α and up-regulated IL-10 expression in response to LPS stimulation.17,18 Reports on CsA-mediated inhibition of the allostimulatory capacity of DCs to T cells are more conflicting.14,15,17 CsA-treated DCs showed decreased capacity to stimulate the proliferation of allogeneic T cells in the presence of TGF-β but not LPS.14 Unexpectedly, we observed a reduced MLR capacity of CsA-treated DCs to allogeneic T cells following LPS maturation in our study. This was not due to “carryover” effects of CsA-treated DCs, because the concentration of remnant CsA was lower than 1 ng/mL and thus unable to influence T-cell proliferation. It is believed that the MLR capacity of matured DCs is dependent on expression of costimulatory molecules (CD80, CD86, and CD40) and MHC molecules (Ia); however, no CsA-mediated changes in the expression of these surface markers were observed in our study, excluding the possibility that inhibition of DC MLR capacity by CsA is due to inhibition of costimulatory molecule expression. An alternative explanation may be that CsA affects the expression of other molecules involved in the one-way MLR capacity of DCs to stimulate allo–T cells—for example, adhesion molecules.
DCs are the antigen-presenting cells most responsible for setting the adaptive immune response in motion, and migration is a crucial aspect of the immunobiology of DCs.20 The interaction of DCs with naive T cells in secondary lymphoid organs is pivotal to the activation of T cells and initiation of adaptive immune responses against exogenous antigens.19,20,23,24 CsA-treated DCs display normal expression of CCR1, CCR2, CCR5, and CCR6 (Figure 4A), which implies that DC infiltration into tissues may be unaffected. This is supported by our observation that CsA-treated mice have similar numbers of CD11c+ cells in the skin epidermis to control animals and that CsA-treated DCs displayed normal responsiveness to MIP-1α (Figure 2A). CsA-treated DCs demonstrated inhibited CCR7 up-regulation upon maturation and were unresponsive to MIP-3β (Figures 2A and 4A). Therefore, it could be inferred that migration of DCs into T-cell areas of draining lymph nodes and spleens would be impaired, which was verified by our in vivo migration assay (Figure 3). The inability of CsA-treated DCs to turn off CCR1, CCR2, CCR5, and CCR6 expression and inability to turn on CCR7 expression upon maturation both contributed to impaired migration of CsA-treated DCs out of skin tissues and into secondary lymphoid organs. We suggest that CsA treatment may disturb interactions between DCs and T cells in secondary lymphoid organ microenvironments by impairing the migration of DCs out of tissues and into spleen and lymph nodes via alteration of CKR expression.
CsA inhibits PGE production by impairing COX gene expression in a variety of cell types.46,47 PGE2 is a potent modulator of the immune response and has been shown to enhance T-cell stimulatory capacity and migratory activity of monocyte-derived DCs (MoDCs). It has been reported recently that stimulation of immature MoDCs with PGE2 alone, or in association with CD40 ligand, TNF-α, LPS, or IL-1β, induces surface expression of CCR7 and acquisition of migratory responsiveness to CCL21/SLC.48,49 As shown in this study, CsA treatment inhibited LPS-induced COX-2 expression and ensuing PGE2 production. When CsA-treated cultures were supplemented with exogenous PGE2, chemokine receptor expression and migratory capacity of CsA-DCs toward MIP-1α and MIP-3β were restored (Figure 5). This strongly suggests that CsA impairs the migratory capacity of DCs by inhibiting the ability of DCs to produce PGE2 in response to LPS, leading to alterations in CKR expression. MAPK signaling and NF-κB–regulated gene transcription, along with NFAT activation, are among the mechanisms affected during CsA-induced immune suppression.2 In our study, we found that CsA reduced the levels of phosphorylated I-κBα and nuclear NF-κB normally induced during DC maturation, suggesting that NF-κB–regulated gene expression may be inhibited (Figure 6). MAPK signaling pathways are involved in the regulation of CKR and COX-2 expression,53-55 and expression of chemokine receptors, including CCR3, CCR7, CXCR1, and CXCR2, are also regulated by NF-κB.56 Therefore, CsA-mediated inhibition of NF-κB nuclear translocation and MAPK activation may be partly responsible for the inhibition of COX-2 and CKR expression observed in CsA-treated DCs.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-07-2412.
Supported by grants from the National Key Basic Research Program of China (2001CB510002) and the National Natural Science Foundation of China (30121002 and 30128022).
T.C. and J.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We sincerely thank Ms Weiqin Ni, Ms Chunfang Luo, and Ms Rui Zhang for their excellent technical assistance and acknowledge the helpful comments of Dr Jane Rayner.