Abstract
Murine mixed hematopoietic chimerism can be achieved following nonmyeloablative conditioning with cyclophosphamide, T cell–depleting monoclonal antibodies, and thymic irradiation. Donor lymphocyte infusions (DLIs) 35 days after bone marrow transplantation (BMT) convert mixed to full donor chimerism and mediate graft-versus-lymphoma effects without graft-versus-host disease. We evaluated the role of T-cell subsets in DLIs in converting mixed to full donor chimerism in a fully major histocompatibility complex–mismatched strain combination. Whereas DLIs administered on day 35 converted 100% of mixed chimeras to full donor chimerism, conversion was less frequent when either CD4 or CD8 cells were depleted, indicating that both subsets contribute to the conversion. Surprisingly, administration of CD8-depleted DLIs led to complete loss of donor chimerism in a high proportion (54%) of recipients compared with CD4-plus CD8-depleted DLIs (15%) or CD4-depleted DLIs (0%) (P < .05). DLIs administered at early time points after BMT (eg, day 21) also precipitated rejection of donor marrow by recipient αβ T cells, in association with donor CD4 cell expansion and high production of interleukin 2 (IL-2), IL-4, and interferon-γ. Thus, DLIs can paradoxically induce marrow rejection by residual host αβ T cells. These results have implications for the timing of and use of subset depletion of DLIs in recipients of nonmyeloablative transplants.
Introduction
In efforts to overcome the toxicities associated with conventional hematopoietic cell transplantation (HCT), nonmyeloablative preparative regimens have been used in various rodent models1 and in clinical trials.2-5 We have used mixed chimerism as immunotherapy for hematologic malignancies in a 2-step process involving establishment of donor-specific tolerance by inducing mixed chimerism with nonmyeloablative conditioning that includes monoclonal antibodies (mAbs), which deplete donor and host T cells in vivo. Mixed chimerism then serves as a platform for adoptive immunotherapy using donor lymphocyte infusions (DLIs), which convert mixed to full donor chimeras, and mediate graft-versus-tumor (GVT) effects without causing graft-versus-host disease (GVHD).6-8 Thus, a lymphohematopoietic graft-versus-host reaction (LGVHR) is used to achieve GVT effects without GVHD.6-8
We have now sought to delineate the T-cell subsets that contribute to the LGVHR mediated by DLI in mixed chimeras produced in the fully major histocompatibility complex (MHC)–mismatched B10.A (H2a) → C57BL/B6 (H2b) strain combination using a cyclophosphamide (Cytoxan; CTX)–based nonmyeloablative conditioning regimen,6 in which durable mixed chimerism and donor-specific immune tolerance is normally achieved.7 DLI administered on day 35 converts these mixed chimeras to full donor chimeras without causing GVHD.6 With this regimen, anti-CD4 and anti-CD8 mAbs in the conditioning regimen circulate at high levels for several weeks following bone marrow transplantation (BMT), resulting in in vivo depletion of T cells in the marrow inoculum.9,10 We have now analyzed the T-cell subsets involved in this conversion by DLIs administered on day 35 and examined the effect of earlier DLI administration on chimerism. The results paradoxically uncover a residual rejection capacity in the host given early DLI or CD8 cell–depleted DLI. Collectively, our results suggest a model wherein graft-versus-host (GVH)–reactive donor T cells may activate residual host T cells and thereby trigger rejection of donor marrow grafts.
Materials and methods
Animals
Female or male B10.A (H2a: Kk, Ik, Dd) donor mice and recipient C57BL/6 (H-2b: Kb, Ib, Db), T-cell receptor β (TCRβ) knockout C57BL/6 and B10.RIII (H-2r: KrIrDr) mice were purchased from Frederick Cancer Research Facility, National Cancer Institute (Frederick, MD) or from the Jackson Laboratory (Bar Harbor, ME). Mice were used for in vivo experiments at 8 to 12 weeks of age. All mice were housed in autoclaved microisolator environments, and all manipulations were performed in a laminar flow hood.
Preparation of bone marrow cells and spleen cells
Bone marrow was harvested and single-cell suspensions were prepared as described previously.11 Donor spleens were harvested and gently teased in ammonium chloride potassium (ACK) lysing buffer (BioWhittaker, Walk-ersville, MD). Single-cell suspensions were filtered through nylon mesh. Alternatively, single-cell splenocyte suspensions were separated over a Lympholyte-M density gradient (Cedarlane Laboratories, Hornby, ON, Canada).
Transplantation of allogeneic BMCs and SPCs in recipients of nonmyeloablative conditioning
Mixed chimerism was induced in female C57BL/6 (H2b) mice by a nonmyeloablative cyclophosphamide (Cytoxan [CTX])–based regimen as described previously.6 The regimen includes T cell–depleting anti-CD4 mAb GK1.5 (2.0 mg) and anti-CD8 mAb 2.43 (1.4 mg) intraperitoneally on day –5, CTX (Mead Johnson, Princeton, NJ) 200 mg/kg intraperitoneally on day –1, and 7 Gy thymic irradiation (60Co source or Siemens x-ray machine, operated at 250 kVp, 1.47 Gy/min) on day 0. Twenty million B10.A bone marrow cells (BMCs) were injected intravenously on day 0 as described.6 DLI consisted of 30 × 106 (final injected number) donor-type (B10.A) spleen cells (SPCs) that were either untreated, complement treated, or depleted of T cells with anti-CD4 mAb GK1.5 or anti-CD8 mAb 2.43 plus low-toxicity rabbit complement, as described previously.12 T-cell depletion was analyzed by flow cytometry (FCM) and less than 1% residual cells of the depleted subset remained.
In further experiments, male C57BL/6 mice received a modified nonmyeloablative conditioning regimen that included CTX 200 mg/kg given intraperitoneally on day –1 and 7 Gy thymic irradiation on day 0 prior to transplantation of 15 × 106 male B10.A BMCs and a total of 10 × 106 T-cell subset–depleted (with GK1.5 or 2.43 plus sheep antirat IgG magnetic beads [Dynal, Lake Success, NY]) or undepleted B10.A SPCs given intravenously on day 0. The recipient mice were treated with either anti-CD4 mAb GK1.5 (2.0 mg) or anti-CD8 mAb 2.43 (1.4 mg) or both mAbs on day –5, or received no T cell–depleting mAbs.
Assessment of chimerism
Chimerism in white blood cells (WBCs) was assessed as described9,10 by 2-color FCM using a Becton Dickinson FACScan cytometer (Mountain View, CA) and analyzed to determine the levels of donor-type hematopoietic reconstitution. WBCs were prepared and analyzed for lineage chimerism by FCM after staining with biotinylated anti-Dd mAb 34-2-12 (prepared in our laboratory) plus phycoerythrin (PE)–conjugated streptavidin (PEA) or Cychrome streptavidin (CycSA) versus anti–CD4-fluorescein isothiocyanate (FITC), anti–CD8β-FITC, anti–B220-FITC (all purchased from PharMingen, San Diego CA), anti–Mac-1-FITC (Caltag, San Francisco, CA), anti–CD3e-PE, anti–CD4-PE, anti–CD8-PE, anti–B220-PE, or anti–CD11b(Mac-1)–PE (PharMingen). Nonreactive control mAb HOPC1-FITC (mouse IgG2a prepared in our laboratory) and rat IgG2a-PE (Pharmingen) were used as negative controls. To reduce nonspecific binding, 10 μL mAb 2.4G2 (anti–Fcγ-RII/III receptor, CDw32) hybridoma supernatant was added.13 The relative percentage of donor cells in a chimera was calculated using the formula: 100% × (donor phenotype percentage positive-isotype control)/[(donor phenotype positive-isotype control) + (recipient phenotype percent positive-isotype control)]. Propidium iodide (PI) staining and live gating on PI– cells were performed. Ten thousand events were collected and analyzed.
Mixed lymphocyte reactions and cell-mediated lympholysis assays
These were performed by standard techniques as we have previously described.7
ELISA spot assays
The 96-well enzyme-linked immunosorbent assay (ELISA) spot plates (Polyfiltronics, Rockland, MA) were coated with a capture mAb in sterile phosphate-buffered saline (PBS) overnight. Anti–interleukin 2 (IL-2), anti–interferon γ (IFN-γ), and anti–IL-4 capture mAbs were used at 3, 4, and 2 μg/mL, respectively (PharMingen). On the day of the experiment, the plates were washed twice with sterile PBS, blocked for 1.5 hours with PBS containing 1% bovine serum albumin (BSA), then washed 3 times with sterile PBS. Responder cells were added to wells previously filled with stimulator cells as previously described.14 Cells were incubated for different periods of time, depending on the cytokine measurement: 20 hours for IL-2 and 42 hours for IFN-γ and IL-4. The plates were washed 3 times with PBS, then 4 times with PBS containing 0.025% Tween (PBST). Biotinylated antilymphokine detection mAbs were added at 2 μg/mL (PharMingen) and incubated either for 5 hours at room temperature or overnight at 4°C. After washing 3 times with PBST, avidin-horseradish peroxidase (1:2000) was added to each well for 1.5 hours. Four washes with PBS were performed before the spots were revealed by the addition of the developing solution composed of 800 μL 3-amino 9-ethylcarbazole (AEC; Sigma-Aldrich, St Louis, MO; 10 mg dissolved in 1 mL dimethylformamide) in 24 mL 0.1 M sodium acetate, pH 5.0, catalyzed by 12 μLH2O2. The resulting spots were counted and analyzed on a computer-assisted ELISA spot image analyzer (CTL, Cleveland, OH).
Statistical analysis
Survival data were analyzed using the log-rank test. Differences between group weights were tested using Mann-Whitney U test or Student t test of means. The Fisher test was used to compare the incidence of rejection and conversion to full chimerism. A paired t test was used to compare antigen-specific cytokine responses to background in the ELISPOT assay.
Results
Both CD4 and CD8 T cells in DLIs contribute to conversion of mixed to full donor chimerism, but CD8 cells play a predominant role
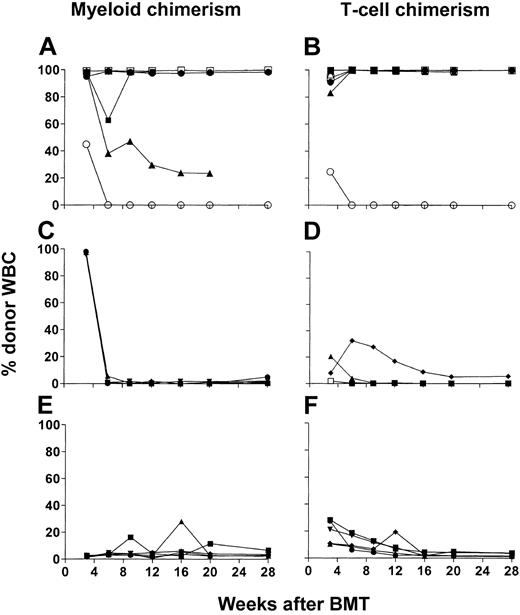
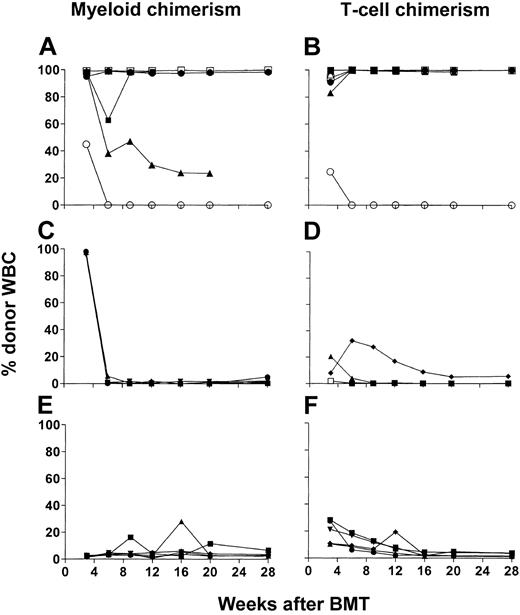
To evaluate the role of T-cell subsets in DLIs administered on day 35 in the conversion from mixed to full donor chimerism, we established mixed chimerism in C57BL/6 mice (H2b) that were conditioned as described6 with our nonmyeloablative regimen followed by administration of 20 × 106 B10.A (H2a) BMCs on day 0. Similar to previous results, the majority (63 of 64, 98.4%) of these animals achieved low levels of mixed hematopoietic chimerism. On day 35, DLIs consisting of 30 × 106 SPCs treated with complement only (C′ control) or depleted in vitro of the CD4 or CD8 T-cell subset were administered. Donor CD4, CD8, B-cell, monocyte, and granulocyte chimerism was assessed in peripheral WBCs until 16 or 37 weeks after BMT, when the animals were killed. Because the granulocyte and monocyte lineages showed similar chimerism levels, as did CD4, CD8, and B cells, donor granulocyte chimerism is depicted in Figure 1 to represent myeloid chimerism and CD4 T-cell data are presented to represent lymphoid lineages. Pooled data are presented from 2 different experiments, each of which produced similar results.
Both CD4 and CD8 T-cell subsets contribute to conversion to full chimerism, whereas CD8 cells play a requisite role. (A-J) Time course of individual donor granulocyte chimerism (representative of both myeloid lineages followed; left panels) and donor CD4+ chimerism (representative of all lymphoid lineages followed; right panels). WBCs chimerism is shown for C57BL/6 (H2b) mice treated with CTX 200 mg/kg on day –1 and 7 Gy thymic irradiation on day 0 that received 20 × 106 BMCs on day 0 and a final number of 30 × 106 T-cell subset–depleted or undepleted SPCs on day 35 after BMT from B10.A (H2a) mice. Recipients were given no DLI (n=9; A-B), complement only–treated DLI (n=11; C-D), CD4 cell–depleted DLI (n=12; E-F), CD8 cell–depleted DLI (n=11; G-H), or CD4 and CD8 cell–depleted DLI (n=20; I-J). Each line represents an individual animal in each panel. Data were pooled from 2 independent experiments.
Both CD4 and CD8 T-cell subsets contribute to conversion to full chimerism, whereas CD8 cells play a requisite role. (A-J) Time course of individual donor granulocyte chimerism (representative of both myeloid lineages followed; left panels) and donor CD4+ chimerism (representative of all lymphoid lineages followed; right panels). WBCs chimerism is shown for C57BL/6 (H2b) mice treated with CTX 200 mg/kg on day –1 and 7 Gy thymic irradiation on day 0 that received 20 × 106 BMCs on day 0 and a final number of 30 × 106 T-cell subset–depleted or undepleted SPCs on day 35 after BMT from B10.A (H2a) mice. Recipients were given no DLI (n=9; A-B), complement only–treated DLI (n=11; C-D), CD4 cell–depleted DLI (n=12; E-F), CD8 cell–depleted DLI (n=11; G-H), or CD4 and CD8 cell–depleted DLI (n=20; I-J). Each line represents an individual animal in each panel. Data were pooled from 2 independent experiments.
WBCs of peripheral blood in the control group receiving no DLIs showed generally stable but low levels of donor chimerism in all lineages (Figures 1A-B and 2A). Complement-treated control DLIs led to conversion to full (100%) donor chimerism in all animals by 11 weeks after BMT (Figures 1C-D and 2A). CD4 cell–depleted (CD4–) DLIs led to a marked increase in donor myeloid and lymphoid chimerism in 7 of 12 animals, and 5 converted to full donor chimerism (Figures 1E-F and 2A). Among the recipients of CD8-depleted (CD8–) DLIs (Figures 1G-H and 2A), only 4 of 11 showed fluctuating increases in donor myeloid and lymphoid chimerism, and none converted to full donor chimerism. One (5%) of 20 animals in the group receiving DLIs depleted of CD4 and CD8 subsets (CD4–8– DLIs) showed conversion to full myeloid and lymphoid donor chimerism (Figures 1I-J and 2A), but the group otherwise showed stable chimerism, similar to controls receiving no DLI. We killed several animals from each experimental group to assess donor engraftment in various organs (bone marrow, spleen, and lymph nodes). FCM analysis showed similar donor chimerism in various lineages to that observed in peripheral WBCs (data not shown). Consistent with our previous results,6 none of the animals showed signs of GVHD in this experiment, weight loss, or death (data not shown). Thus, whereas both CD4 and CD8 cells in DLIs contribute to conversion to full chimerism, CD8 cells play a predominant role, and this conversion occurs without observable GVHD when DLIs are given 35 days after BMT.
CD4 cells given with CD8 cells in day-35 DLIs promote conversion to full donor chimerism, but CD4 cells given without CD8 cells promote loss of chimerism. The percentage is shown of animals with conversion to full donor chimerism (A) or with loss of detectable donor chimerism (B) following various DLIs. The same animals presented in Figure 1 are presented here. Recipients were given no DLI (n=9; group A, No DLI), complement only–treated DLI (n=11; group B, DLI + c), CD4 cell–depleted DLI (n=12; group C, CD4 depl. DLI), CD8 cell–depleted DLI (n=11; group D, CD8 depl. DLI), or CD4 and CD8 cell–depleted DLI (n=20; group E, CD4 + 8 depl. DLI). Each bar represents one group in each panel. Data were pooled from 2 independent experiments. The difference in percentages of animals with conversion to full chimerism was statistically significant for group E versus group B or group C; for group C versus group D and for group B versus group D. The difference in percentages of animals with loss of chimerism was statistically significant for group D versus group B, group C, and group E. In the 2 separate experiments, the following numbers of mice converted to full donor chimerism in experiments 1 and 2, respectively: group A, 0 of 6, 0 of 3; group B, 8 of 8, 3 of 3; group C, 3 of 4, 2 of 8; group D, 0 of 4, 0 of 7; group E, 1 of 15, 0 of 5. In the 2 separate experiments, the following numbers of mice lost chimerism in experiments 1 and 2, respectively: group A, 1 of 6, 1 of 3; group B, 0 of 8, 0 of 3; group C, 0 of 4, 0 of 8; group D, 2 of 4, 4 of 7; group E, 2 of 15, 1 of 5.
CD4 cells given with CD8 cells in day-35 DLIs promote conversion to full donor chimerism, but CD4 cells given without CD8 cells promote loss of chimerism. The percentage is shown of animals with conversion to full donor chimerism (A) or with loss of detectable donor chimerism (B) following various DLIs. The same animals presented in Figure 1 are presented here. Recipients were given no DLI (n=9; group A, No DLI), complement only–treated DLI (n=11; group B, DLI + c), CD4 cell–depleted DLI (n=12; group C, CD4 depl. DLI), CD8 cell–depleted DLI (n=11; group D, CD8 depl. DLI), or CD4 and CD8 cell–depleted DLI (n=20; group E, CD4 + 8 depl. DLI). Each bar represents one group in each panel. Data were pooled from 2 independent experiments. The difference in percentages of animals with conversion to full chimerism was statistically significant for group E versus group B or group C; for group C versus group D and for group B versus group D. The difference in percentages of animals with loss of chimerism was statistically significant for group D versus group B, group C, and group E. In the 2 separate experiments, the following numbers of mice converted to full donor chimerism in experiments 1 and 2, respectively: group A, 0 of 6, 0 of 3; group B, 8 of 8, 3 of 3; group C, 3 of 4, 2 of 8; group D, 0 of 4, 0 of 7; group E, 1 of 15, 0 of 5. In the 2 separate experiments, the following numbers of mice lost chimerism in experiments 1 and 2, respectively: group A, 1 of 6, 1 of 3; group B, 0 of 8, 0 of 3; group C, 0 of 4, 0 of 8; group D, 2 of 4, 4 of 7; group E, 2 of 15, 1 of 5.
Administration of donor CD4 cells without CD8 cells in DLIs paradoxically induces loss of donor chimerism
Surprisingly, we observed a complete loss of donor chimerism (to ≤ 0.7% donor WBCs following previously stable chimerism) in a high percentage (54.4%, 6 of 11) of recipients of CD8– DLIs (Figures 1G-H and 2B). The incidence of loss of chimerism in the group not receiving any DLI was only 22.2% (2 of 9; Figures 1A-B and 2B). In mice receiving CD4–8– DLIs, loss of donor chimerism occurred in only 15% (3 of 20) of animals (Figures 1I-J and 2B; P < .05 compared with CD8– DLI group). Loss of chimerism was not seen in recipients of CD4– DLIs (0 of 12; Figures 1E-F and 2B), or in recipients of C′ control DLIs (0 of 11; Figures 1C-D and 2B; P < .05 compared with recipients of CD8– DLIs). In animals that lost detectable chimerism in WBCs, chimerism was also undetectable in the bone marrow, spleen, and lymph node cells when the animals were killed 37 weeks after BMT (2, 5, or 6 animals/group, data not shown).
The significantly higher incidence of loss of chimerism in recipients of CD8– DLIs compared with recipients of CD4–8– DLIs (P < .05) demonstrates that donor CD4 cells administered without CD8 cells in DLIs induce loss of donor chimerism.
Unseparated DLIs administered on day 21 induce loss of donor chimerism
We compared the effect of DLIs administered at various times in groups of stable chimeras in the B10.A → B6 strain combination. As in previous experiments, animals not receiving DLIs remained chimeric for the duration of follow-up (Figure 3A left panels). DLIs administered on day 35 led to conversion to full donor chimerism (Figure 3A right panels). Strikingly, DLIs administered on day 21 led instead to a complete loss of chimerism in 5 of 6 recipients (Figure 3A middle panels). Similar results were seen with day 21 DLIs in 5 of 5 experiments. A total of 23 (72%) of 32 mice lost measurable chimerism completely after these DLIs compared with only 5 (15%) of 33 non-DLI recipients (P < .0005). Animals that lost chimerism following day-21 DLIs also failed to demonstrate long-term tolerance in mixed lymphocyte reactions (MLRs) and cell-mediated lympholysis (CML) assays performed as described7 at the time the animals were killed, whereas control chimeras showed specific tolerance in these assays (data not shown).
Effect of timing of DLI on changes in chimerism. (A) Loss of chimerism induced by day-21 DLI. Results of one of 5 similar experiments are shown. Each line represents an individual animal; 6 animals/group. (B) Requirement for host αβ T cells for loss of chimerism induced by day-21 DLI. Means ± SD are shown. Wild-type recipients (B6): No DLI, n=6; DLI (30 × 106 SPCs), n=8. TCRβ KO (TCR–/–) recipients: No DLI, n=7; DLI (30 × 106 SPCs), n=7. *Denotes statistically significant difference between DLI and no DLI groups.
Effect of timing of DLI on changes in chimerism. (A) Loss of chimerism induced by day-21 DLI. Results of one of 5 similar experiments are shown. Each line represents an individual animal; 6 animals/group. (B) Requirement for host αβ T cells for loss of chimerism induced by day-21 DLI. Means ± SD are shown. Wild-type recipients (B6): No DLI, n=6; DLI (30 × 106 SPCs), n=8. TCRβ KO (TCR–/–) recipients: No DLI, n=7; DLI (30 × 106 SPCs), n=7. *Denotes statistically significant difference between DLI and no DLI groups.
To determine whether or not host T cells were responsible for the paradoxical loss of chimerism that occurred when DLIs were given on day 21, we compared the effect of these DLIs in mixed chimeras prepared in wild-type B6 mice to that in chimeras prepared in TCRβ-deficient (TCRβ knockout, KO) recipients, which completely lack αβ TCR+ T cells.15 As is shown in Figure 3B, multilineage mixed chimerism developed to similar levels prior to DLI in wild-type and TCRβ KO B6 mice. DLI again led to a complete loss of chimerism in wild-type B6 mice. In contrast, TCRβ KO mice showed an increase in chimerism after 5 weeks, and this pattern was not influenced by the administration of DLIs on day 21 (not significant [NS] at all time points except B-cell chimerism at 5 weeks, P = .04). All TCR KO mice remained chimeric in marrow and spleen when killed 22 or 30 weeks after BMT. No difference in chimerism was seen between TCR KO chimeras that did or did not receive DLIs (eg, mean ± SD, 14.4% ± 4.6% B-cell chimerism in non-DLI versus 17.0% ± 13.0% in DLI group). Among wild-type chimeras, only non-DLI recipients showed any chimerism at death (5 of 8 chimeric; mean ± SD, 6.3% ± 5.0% B-cell chimerism), and the level was significantly lower than that in TCR KO chimeras (P < .05). Thus, we conclude that recipient αβ T cells are responsible for the loss of chimerism that occurs following day-21 DLIs in wild-type B6 recipients.
Alloresponses in the first 5 weeks after BMT in control chimeras and day-21 DLI recipients
The above results suggested that T cells capable of rejecting the donor were present in mixed chimeras 21 to 35 days after BMT. We therefore evaluated alloreactivity by preparing mixed chimeras and killing them on day 21, 28, or 35 to evaluate CML, MLR, and ELISPOT responses to donor, host, and third-party antigens, and to determine the effects of day-21 DLIs on these responses.
On day 21, antidonor, antihost, and anti–third-party MLR responses of splenocytes in 2 of 5 mice not receiving DLIs were similar to those of naive B6 (host-type) mice, whereas 3 chimeras were globally unresponsive. By day 28, chimeras that did not receive DLIs showed unresponsiveness to donor and host, with absent or poor responses to third-party antigens. Even by day 35, poor responses to third-party antigens were seen (data not shown).
Day 21 DLI was associated with the presence of measurable antihost MLR responses in 3 of 5 animals, antidonor responses in 1 of 5, and anti–third-party responses in 4 of 5 mice on day 28. By day 35, 1 of 4 DLI recipients responded to all 3 stimulators, and the remaining 3 were generally unresponsive (data not shown).
We also performed CML assays on the day-35 splenocyte populations. The non-DLI chimeras were all unresponsive to donor, host, and third-party antigens (data not shown), consistent with the poor recovery of CD8 T cells seen at 4 weeks (Table 1). Whereas 3 of 4 DLI recipients produced similar results, one DLI recipient showed a weak, but measurable antidonor CML response (percent specific lysis 17.7% at 100:1 responder-target ratio), despite the absence of an anti–third-party CML response (data not shown).
The above data did not strongly implicate a cytotoxic T-lymphocyte (CTL)–mediated effector mechanism of rejection of donor marrow in recipients of day-21 DLIs, suggesting that cytokines might instead be responsible. Results of ELISPOT assays performed 7 days following DLI (day 28) were consistent with this possibility.
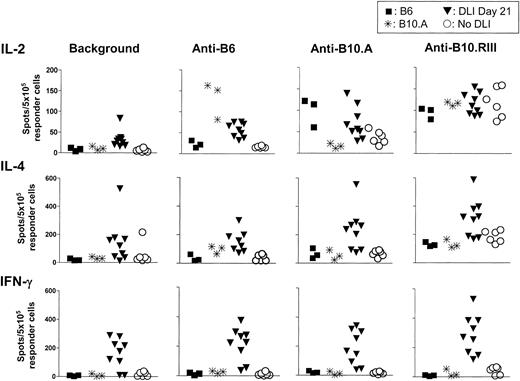
As shown in Figure 4, day-28 spleens of control (non-DLI) mixed chimeras (n = 6) did not respond above baseline (background) to B6 (host-type) stimulators. However, a weak (compared with that of naive B6 mice) but significant IL-2 response to donor antigens was detected (P < .05). Strong anti–third-party IL-2 responses above baseline were detected (P < .001), and these were similar to the responses of naive B6 and B10.A mice. IL-4 responses of chimeras to third-party antigens were significantly greater than baseline (P < .01 by paired t test) and were also similar to those of naive B6 and B10.A controls, whereas the chimeras were specifically unresponsive to donor and host antigens. A weak but significant IFN-γ response to third-party stimulators (P < .05 versus baseline) was detected among chimeras, which demonstrated complete unresponsiveness to donor and host antigens. In summary, the non-DLI chimeras demonstrated specific cytokine hyporesponsiveness (with a weak antidonor IL-2 response) or complete, specific unresponsiveness (IFN-γ, IL-4) toward the donor and host by day 28.
ELISPOT analysis of cytokine production in response to donor (B10.A), host (B6), and third-party (B10.RIII) alloantigens by day 28 splenocytes of mixed chimeras receiving no DLI or DLIs on day 21 after BMT. Results of 2 similar experiments are shown. Each symbol represents an individual animal. The upper panel shows IL-2–producing cells, the middle panel shows IL-4–producing cells, and the lower panel shows IFN-γ–producing cells among SPCs from control naive B6 mice (n=3; ▪), naive B10.A mice (n=3; *), chimeras receiving DLI on day 21 (DLI day 21; n=9; ▾) or not receiving DLI (no DLI; n=6; ○). Chimeric SPCs were collected on day 28. The in vitro stimulators are listed from left to right: medium (background in the absence of stimulator cells in vitro), B6, B10.A, and B10.RIII.
ELISPOT analysis of cytokine production in response to donor (B10.A), host (B6), and third-party (B10.RIII) alloantigens by day 28 splenocytes of mixed chimeras receiving no DLI or DLIs on day 21 after BMT. Results of 2 similar experiments are shown. Each symbol represents an individual animal. The upper panel shows IL-2–producing cells, the middle panel shows IL-4–producing cells, and the lower panel shows IFN-γ–producing cells among SPCs from control naive B6 mice (n=3; ▪), naive B10.A mice (n=3; *), chimeras receiving DLI on day 21 (DLI day 21; n=9; ▾) or not receiving DLI (no DLI; n=6; ○). Chimeric SPCs were collected on day 28. The in vitro stimulators are listed from left to right: medium (background in the absence of stimulator cells in vitro), B6, B10.A, and B10.RIII.
Strikingly, the chimeric animals receiving DLIs on day 21 (n = 9) showed significantly increased baseline IL-2–producing (P < .01) and IFN-γ–producing (P < .005) cells compared with non-DLI controls. No significant difference was detected between the groups in baseline IL-4–producing cell numbers. Although average IL-2–producing cell numbers did not increase significantly in response to donor or host antigens, paired analysis revealed significant responses to donor, host, and third-party antigens compared with the baseline (P < .05, < .005, < .0001, respectively) in the absence of in vitro stimulation. A similar situation prevailed for IL-4–producing cells (P < .005, < .01, < .0001 by paired t test for responses to donor, host, and third-party antigens), but not for IFN-γ, in which significant (P < .05) responses to third-party but not donor or host antigens were detectable by paired comparison to baseline values. In summary, day-21 DLIs led to a marked increase in IL-2– and IFN-γ–producing cells, with clear evidence for loss of tolerance to host and donor antigens for IL-2– and IL-4–producing cells.
An analysis of splenic chimerism in the groups analyzed on day 28 revealed an absence of donor CD8 cells and very few host CD8 cells, and the presence of relatively high percentages of donor and recipient CD4 cells (Table 1). DLIs on day 21 did not lead to a change in B-cell or CD8 T-cell chimerism, but did lead to a significant (P < .005) increase in the percentage of donor CD4 cells in the spleen on day 28. Absolute numbers of donor CD4 cells (P < .01) but not of recipient CD4 cells or of donor or recipient CD8 cells were increased in DLI recipients compared with controls. These results are consistent with the possibility that donor CD8 cells in day-21 DLIs were depleted by residual anti-CD8 mAb in the serum, whereas DLI CD4 cells were not depleted and were able to proliferate as a result of the GVH alloresponse. By FCM analysis, anti-CD4 and anti-CD8 mAbs were both present in the undiluted serum on day 21 at levels that saturated all mAb-binding sites on normal splenocytes (data not shown).
Evidence that donor CD4 cells have potential to inhibit engraftment of marrow given on day 0
We next examined the effect of donor and recipient T-cell subsets present at the time of BMT on engraftment and GVHD. For this purpose the conditioning regimen was modified so that mice received either no T-cell–depleting mAbs, or only anti-CD4, anti-CD8, or both mAbs on day –5, and donor SPCs were administered on day 0. B6 mice received CTX 200 mg/kg on day –1, 7 Gy thymic irradiation on day 0, and 15 × 106 BMCs and 10 × 106 SPCs from B10.A donors on day 0.
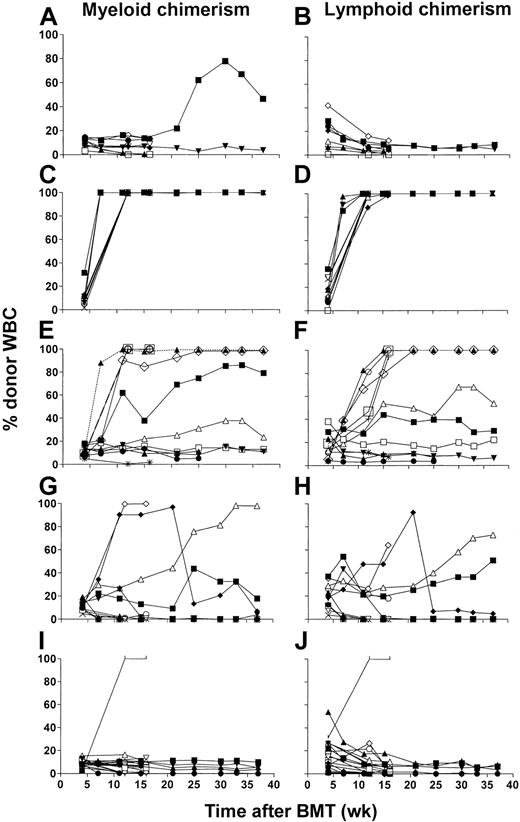
Control recipients (n = 5) of donor SPCs on day 0 without T cell–depleting mAbs showed no donor engraftment at any time (data not shown) and remained healthy with continuous weight gain (not shown). In contrast, among recipients of CD4-depleting mAb in vivo (n = 6), nearly complete or complete donor myeloid chimerism (95%-99%) was achieved by week 3 after BMT in 5 of 6 mice (Figure 5A-B). Full chimerism was sustained long-term in 3 of the 5 mice. One animal died early of an anesthetic overdose and 2 animals died, at 12 and 20 weeks after BMT, with 20% to 30% body weight loss and clinical signs of GVHD (ruffled fur, skin lesions). Among the 3 remaining animals, the 2 with nearly complete donor chimerism also had weight loss and clinical evidence of GVHD, whereas the nonchimeric animal remained healthy and gained weight (data not shown).
In vivo CD4 depletion of BMCs and DLIs administered on day 0 facilitate full donor myeloid and lymphoid engraftment. (A-F) Time course of individual MAC-1+ donor myeloid cell (left panels) and CD3+ donor T cell (right panels) chimerism in WBCs of B6 (H2b) mice treated with CTX 200 mg/kg on day –1 and 7 Gy thymic irradiation on day 0 followed by 15 × 106 BMCs and 10 × 106 SPCs on day 0 from B10.A (H2a) donor mice. Five days before BMT, recipients were given anti-CD4 mAb alone (n=6; A-B), anti-CD8 mAb alone (n=6; C-D), or anti-CD4 and anti-CD8 mAbs (n=5; E-F). Each line represents an individual animal in each panel. One of 2 experiments is presented.
In vivo CD4 depletion of BMCs and DLIs administered on day 0 facilitate full donor myeloid and lymphoid engraftment. (A-F) Time course of individual MAC-1+ donor myeloid cell (left panels) and CD3+ donor T cell (right panels) chimerism in WBCs of B6 (H2b) mice treated with CTX 200 mg/kg on day –1 and 7 Gy thymic irradiation on day 0 followed by 15 × 106 BMCs and 10 × 106 SPCs on day 0 from B10.A (H2a) donor mice. Five days before BMT, recipients were given anti-CD4 mAb alone (n=6; A-B), anti-CD8 mAb alone (n=6; C-D), or anti-CD4 and anti-CD8 mAbs (n=5; E-F). Each line represents an individual animal in each panel. One of 2 experiments is presented.
In contrast to these results, in vivo depletion of CD8+ cells (n = 6) was associated with sustained, low-level donor engraftment in only 1 of 6 mice (Figure 5C-D). One animal with 38.4% weight loss prior to death at 4 weeks after BMT was not assessed for chimerism. All other animals survived without weight loss and remained clinically healthy (data not shown).
All recipients of combined CD4- and CD8-depleting mAbs in vivo (n = 5) engrafted with low donor myeloid (2%-6.7%; Figure 5E) and lymphoid chimerism (1.6%-3.9%; Figure 5F), which persisted until at least week 28 after BMT. Thus, sustained donor engraftment occurred most frequently in association with in vivo depletion of CD4 plus CD8 cells, but a high incidence was also seen in recipients depleted only of CD4 cells. Engrafted animals in the latter group showed high levels of donor chimerism, and this condition was associated with clinical evidence of GVHD. In contrast, the combined depletion of CD4 and CD8 T cells permitted mixed chimerism to be achieved in the absence of clinically apparent GVHD.
In a repeat experiment, 5 of 8 recipients treated with no mAb showed no chimerism, whereas the remaining 3 showed low levels (< 15%) of myeloid and lymphoid mixed chimerism at 3 weeks, which disappeared by 6 weeks. In contrast, most animals (6 of 7) treated with anti-CD4 mAb showed low (< 20% donor) myeloid and lymphoid chimerism at 3 weeks (P < .05 versus no mAb group for B cells, monocytes, and granulocytes), which persisted at 6 weeks and disappeared by week 9. These mice remained healthy. Only 1 of 8 recipients of anti-CD8 mAb showed initial low (≤ 5%) donor myeloid chimerism (NS versus no mAb group for any lineage), which was lost by 6 weeks. Eight of 8 mice treated with anti-CD4 plus anti-CD8 showed a low level (eg, mean 6% donor B cells at 6 weeks) of chimerism. All animals showed weight gain, with no clinical evidence of GVHD (data not shown). Thus, although no full chimerism or GVHD was seen in this experiment, the observation that in vivo CD4 depletion increased donor engraftment compared with no mAb treatment or anti-CD8 mAb treatment was reproduced.
The results described, in which CD4 depletion in vivo was required to permit sustained engraftment of donor marrow in most animals, suggested that recipient CD4 cells might be capable of rejecting donor marrow, even in the absence of CD8 cells. However, given that the in vivo depletion affected both donor and host CD4 cells, our results did not rule out the possibility that donor CD4 cells might paradoxically inhibit donor engraftment, in a manner analogous to the effects described with day-35 DLIs. We therefore evaluated engraftment in mice not receiving in vivo T-cell depletion, but receiving B10.A BMCs and a total of 10 × 106 donor SPCs that were or were not depleted of CD4 or CD8 T cells following treatment with CTX 200 mg/kg on day –1 and 7 Gy thymic irradiation on day 0.
The control animals receiving CTX, thymic irradiation, BMCs, and untreated SPCs (n = 5) on day 0 again showed no donor engraftment. Five of 5 animals receiving CD4 cell–depleted SPCs on day 0 developed chimerism, at high (> 95% donor) levels in 3 mice and at lower levels in the other 2. Clinical GVHD was not apparent. None of 5 recipients of CD8-depleted SPCs showed any chimerism, nor did any recipients of CD4-depleted plus CD8-depleted SPCs on day 0. In a repeat experiment involving 8 animals per group, however, none of the mice in any group developed measurable chimerism at any time. Thus, although there was variability from experiment to experiment, these results suggest that donor CD8 cells given on day 0 are able to promote donor engraftment in recipients not depleted of either CD4 or CD8 T cells. However, the presence of donor CD4 cells in these inocula may interfere with this engraftment-promoting ability of donor CD8 cells.
Discussion
We have previously demonstrated that GVH alloresponses induced by DLI can be confined to the lymphohematopoietic system, where they can convert mixed to full donor chimerism and mediate GVT effects without causing GVHD.6,8,16,17 We have now performed studies to identify the T-cell subset(s) responsible for this conversion in the setting of a full MHC mismatch in mice undergoing nonmyeloablative BMT. Our data demonstrate that both CD4 and CD8 cells contribute to conversion to full chimerism following day-35 DLI. CD8 cells play a requisite role in this conversion and can mediate conversion even when CD4 cells are depleted. Nevertheless, CD4 T cells significantly increased the frequency with which conversion to full chimerism occurred following DLI, indicating a contribution of CD4 cells to this process.
In addition, these studies have revealed a paradoxical effect of CD4 cells in DLIs. When CD4 cells were given in day-35 DLIs without CD8 cells, disappearance of measurable chimerism was induced in the majority of animals. This effect was dependent on the presence of CD4 T cells in the DLIs because chimerism was not affected by the administration of CD4-depleted plus CD8 cell–depleted DLI. This result demonstrates that provision of donor antigen-presenting cells (APCs) in DLIs is not responsible for inducing loss of chimerism, because the incidence of long-term stable chimerism was similar in recipients of no DLIs and those receiving CD4-depleted plus CD8 cell–depleted DLIs.
Studies involving the administration of donor lymphocytes on day 0, although somewhat variable from experiment to experiment, are consistent with the interpretation that donor CD4 cells can impair engraftment in the presence of residual host immunity. In 2 of 2 experiments, administration of anti-CD4 mAb in vivo without anti-CD8 mAb increased the level of engraftment over that achieved in controls not receiving mAb and that in recipients of anti-CD8 mAb alone. Thus, donor CD8 T cells have the potential to overcome the resistance imposed by recipient T cells, which were largely CD8+ in mice receiving CD4 cell–depleting mAb. Results of studies involving administration of ex vivo T-cell subset–depleted allogeneic SPCs on day 0 to mice that did not receive any mAbs in vivo, though also variable, are consistent with the interpretation that donor CD8 cells have the potential to overcome host resistance mediated by both CD4+ and CD8+ recipient T cells, and that donor CD4 cells interfere with this engraftment-promoting ability of donor CD8 cells. The variable outcomes (engraftment and GVHD) within and between experiments involving donor SPC administration on day 0 to mice not receiving both anti-CD4 and anti-CD8 mAbs in their conditioning suggests that the balance between host-versus-graft (HVG) and GVH responses is very fine in this setting and can be tipped in one or the other direction by unknown variables. In contrast, engraftment is much more reliably achieved, and without GVHD, in mice receiving both CD4-depleting and CD8-depleting mAbs as part of their conditioning.
One possible mechanism for the deleterious effect of donor CD4 cells on donor graft survival under the above conditions is that the GVH reaction induced by CD4 cells provides “help” that activates residual nontolerant host lymphocytes to reject donor marrow. Evidence for the presence of such recipient cells was obtained in ELISPOT assays in non-DLI recipients, in which a significant, though weak, IL-2 response to the donor was detectable on day 28. DLIs given at 21 days after BMT induced loss of chimerism, and this rejection, which was shown to require αβ T cells, was associated with increased IFN-γ– and IL-2–producing effector cells in freshly isolated splenocyte populations. Additionally, IL-2 and IL-4 assays demonstrated a loss of hyporesponsiveness or tolerance to both donor and host in DLI recipients, consistent with the existence of an activated, bidirectional alloresponse in these mice. Some animals also showed antidonor and antihost MLR responses. Although anti-CD8 and anti-CD4 mAbs were still circulating at high levels at this early time point of DLI (day 21), our previous studies have shown more rapid clearance of anti-CD4 than anti-CD8 mAbs in animals receiving this mAb regimen.9 The observation that donor CD4 cells but not CD8 cells were expanded on day 28 in spleens of mice that received day-21 DLI (Table 1) suggests that CD8 cells in the DLI were depleted by the residual circulating mAbs, whereas DLI CD4 cells survived and proliferated in the GVH reaction. The MLR responses and cytokine production detected in response to recipient (in addition to donor) antigens in these mice is consistent with the response of a GVH-reactive Th cell population in the DLI, which may have helped to trigger the HVG-reactive Th population that produced cytokines in response to the donor. Evidence does not suggest a cytolytic mechanism of donor marrow rejection following early DLIs, because CTL responses to the donor were not detectable in most cases on day 35, and very few recipient CD8 T cells were detected in the spleens by day 28. The ELISPOT and MLR data favor a cytokine-mediated mechanism of donor marrow rejection, and this may be a counterpart of the IFN-γ–dependent pathway that has been reported to be capable of destroying host hematopoiesis under other conditions.18 The pathway by which the CD4-mediated GVH reaction elicits this HVG response remains to be elucidated.
In other experiments (data not shown), we have observed that DLIs administered on day 25 can also cause a complete loss of chimerism in a strain combination (B10.BR → BALB/c) in which day 35 DLI converts the animals to full donor chimerism. Although long-term mixed chimeras prepared with this nonmyeloablative regimen are systemically and specifically tolerant of their donors by an intrathymic deletional mechanism,7 the initial depletion of recipient T cells with this conditioning regimen may be incomplete. Additional mechanisms, such as anergy and regulation, may evolve over time (> 3.5 weeks) to result in the tolerization of residual host T cells, resulting in an initially precarious state of tolerance. If this state is left unperturbed, tolerance ultimately ensues.
The observation that CD8 cell–depleted DLIs on day 35 can precipitate loss of chimerism in recipients undergoing nonmyeloablative BMT may have clinical relevance, because CD8-cell depletion has been evaluated in efforts to achieve graft-versus-lymphoma (GVL) without GVHD from DLI in recipients of HLA-identical donor transplants.19,20 Conversion of mixed to full donor chimerism was observed in these studies.21 However, we have not evaluated the effect of subset-depleted DLIs in mice undergoing myeloablative BMT, in which host resistance to the donor may be more fully eliminated than in our CTX-based nonmyeloablative regimen. Furthermore, we have not evaluated the effect of T-cell subset depletion of DLI in MHC-identical murine combinations because naive DLIs are incapable of converting mixed to full donor chimeras in recipients of MHC-matched, multiple minor antigen-mismatched marrow with this nonmyeloablative conditioning regimen in some strain combinations (Y.-M.K. and M.S., manuscript in preparation; J.D.D. et al, unpublished data, August 2001).
Several groups have previously demonstrated that donor CD8 cells can help to promote marrow engraftment in mice,11,22-24 and that this effect is due, in large part but not entirely, to the ability of GVH reactive CD8 T cells to destroy host lymphocytes that otherwise resist engraftment.25 Consistently, patients receiving CD8 cell–depleted bone marrow have shown a higher graft rejection rate than recipients of unmodified marrow.26 However, in sublethally irradiated mice receiving BM transplants from donors differing only at a class II MHC locus, donor CD4+ T cells can eliminate host hematopoietic cells via an IFN-γ–dependent mechanism,18 causing death due to hematopoietic failure.27 Our studies provide the first demonstration that, in the presence of residual host immune resistance to the donor, donor CD4 cells can paradoxically help to trigger the rejection process. Nevertheless, in established mixed chimeras, when CD4 T cells are given along with CD8 cells in DLIs, they help to promote conversion to full donor chimerism.
In recipients of donor SPCs on day 0 with in vivo CD4 depletion, animals with full chimerism all showed clinical evidence of GVHD. This did not occur in animals without engraftment or in sustained chimeras produced via in vivo depletion of both CD4 and CD8 cells, suggesting that CD8 cells given on day 0 may have caused GVHD. No GVHD has ever been observed in recipients of 3-fold larger DLIs on day 35 in this strain combination in these or previous studies.6 The ability to induce GVHD with CD8 T cells administered on day 0 but not with such cells administered in DLIs on day 35 is further evidence of the importance of host recovery from conditioning in conferring resistance to GVHD by DLI.
Thus, the studies here introduce a new concept in HCT. In recipients in which significant HVG alloreactivity persists, the administration of GVH-reactive donor CD4 cells may induce donor graft rejection. Interactions between GVH- and HVG-reactive T cells must now be considered in evaluating the immunobiology of nonmyeloablative HCT. Further studies are needed to dissect the mechanism of this effect and evaluate it in the setting of various histoincompatibilities, which may have important implications for nonmyeloablative HCT and the use of T-cell subset–depleted DLIs in attempts to achieve GVL effect without GVHD.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-02-0643.
Supported by National Institutes of Health grants RO1 CA79989, RO1 HL49915, RO1 EY13310, and RO1 AI33704; in part by Mildred-Scheel Stiftung of the Deutsche Krebshilfe (Y.-M.K.); by a grant from Deutsche Forschungsgemeinschaft (DFG Ma-1664/2-1; M.Y.M.); and by a fellowship from the Foundation pour la Recherche Medicale (F.B.). Y.-M.K. and M.Y.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Robin Laber for expert assistance with the manuscript, Drs Thomas Spitzer and Ronjon Chakraverty for critical reading of the manuscript, and Mr Orlando Moreno and Mr Peter Morgan for technical assistance and expert oversight of animal care.