Abstract
For circulating lymphocytes to migrate to inflammatory sites, they must first adhere to the target tissue endothelium with sufficient strength to overcome the shear forces of blood flow. We previously reported that dermal papillary vessels in acute graft-versus-host disease (aGVHD) support shear-resistant lymphocyte adherence. We now identify the relevant adhesion molecule(s) directing this binding, showing that interactions between lymphocyte CD44 and hyaluronic acid (HA) expressed on dermal vessels in aGVHD alone confer this shear-resistant attachment. Native HA deposits on vascular endothelium support lymphocyte adherence, whereas HA immobilized on plastic does not. HA expressed at dermal endothelium in aGVHD is thus specialized to support lymphocyte adherence under flow conditions, and CD44-HA interactions may contribute to lymphocytotropism to skin in aGVHD.
Introduction
To elucidate the molecular mediators of lymphocyte trafficking in acute graft-versus-host disease (aGVHD), we previously performed adhesion assays under blood flow shear stress conditions1 to analyze lymphocyte binding to endothelia of skin eruptions after allogeneic and autologous hematopoietic stem cell transplantation (HSCT).2 These studies showed that papillary dermal vessels in aGVHD reactions, but not in most other post-HSCT skin eruptions, support shear-resistant adherence of lymphocytes.2 We show here that this binding interaction is mediated by endothelial deposits of hyaluronic acid (HA) specialized to support CD44-dependent lymphocyte adherence. These findings offer new perspectives on the molecular basis of cutaneous aGVHD reactions and highlight a role for HA in the pathobiology of this condition.
Study design
Lymphocyte-skin adherence assay
All specimens were obtained under institutional review board (IRB)–approved protocols. Biopsy samples of involved skin were obtained from 42 recipients of allogeneic HSCT with cutaneous aGVHD, and normal skin was from discarded tissue from cosmetic surgery. The lymphocyte-skin adherence assay was performed using lymphocytes (peripheral blood mononuclear cells [PBMCs]) from healthy donors.2,3
Chemical, enzymatic, and anti-CD44 antibody treatments
For all treatments, adherence assays were performed on alternating sequential sections (treated, or buffer/isotype-control). Lymphocytes were counted in comparable consecutive areas of the papillary dermis using an ocular grid. Protease digestions, EDTA (ethylenediaminetetraacetic acid), and neuraminidase treatments were performed as described.4 Digestion of matrix elements and monoclonal antibody (mAb) (Hermes-1)5 treatments were performed as described in Figure 1.
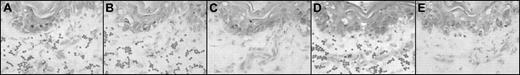
Photomicrographs of sequential sections after skin biopsy from a patient with acute cutaneous GVHD: enzyme and antibody treatments. Representative adherence assay results of experimental manipulations in sequential sections. (A-E) Methyl green-thionin stain; original magnification × 40. Digestion of matrix elements was performed for 30 minutes at 37°C with the following: heparitinase 2 from Flavobacterium heparinum (Calbiochem, La Jolla, CA), 5 mU/mL in buffer (100 mM Na acetate,10 mM Ca acetate, pH 7), keratanase from Pseudomonas species (Calbiochem), 0.5 U/mL in buffer (50 mM Tris HCl, pH 8), hyaluronidase from Streptomyces hyalurolyticus (Sigma, St.Louis, MO), 20 U/mL in PBS buffer. For antibody treatments, assays were performed on alternating sequential sections in the presence of the function-blocking mAb Hermes-1 (10 μg/mL; gift from Dr Brenda Sandmaier, Fred Hutchinson Cancer Research Center) or rat IgG2a isotype antibody (control). Note the characteristic appearance of arcuate, palisading adherent lymphocytes (dark dots) binding to dermal papillary structures (as shown in panels A, B, and D). (A) Heparitinase 2 treatment of skin section. (B) No enzyme treatment; section incubated with PBS (matched control). (C) Hyaluronidase treatment of skin section. (D) Keratanase treatment of skin section. (E) Lymphocytes incubated with anti-CD44 mAb Hermes-1. Notice the absence of binding in the section treated with hyaluronidase (C) and in the section in which the assay was performed in the presence of Hermes-1 (E).
Photomicrographs of sequential sections after skin biopsy from a patient with acute cutaneous GVHD: enzyme and antibody treatments. Representative adherence assay results of experimental manipulations in sequential sections. (A-E) Methyl green-thionin stain; original magnification × 40. Digestion of matrix elements was performed for 30 minutes at 37°C with the following: heparitinase 2 from Flavobacterium heparinum (Calbiochem, La Jolla, CA), 5 mU/mL in buffer (100 mM Na acetate,10 mM Ca acetate, pH 7), keratanase from Pseudomonas species (Calbiochem), 0.5 U/mL in buffer (50 mM Tris HCl, pH 8), hyaluronidase from Streptomyces hyalurolyticus (Sigma, St.Louis, MO), 20 U/mL in PBS buffer. For antibody treatments, assays were performed on alternating sequential sections in the presence of the function-blocking mAb Hermes-1 (10 μg/mL; gift from Dr Brenda Sandmaier, Fred Hutchinson Cancer Research Center) or rat IgG2a isotype antibody (control). Note the characteristic appearance of arcuate, palisading adherent lymphocytes (dark dots) binding to dermal papillary structures (as shown in panels A, B, and D). (A) Heparitinase 2 treatment of skin section. (B) No enzyme treatment; section incubated with PBS (matched control). (C) Hyaluronidase treatment of skin section. (D) Keratanase treatment of skin section. (E) Lymphocytes incubated with anti-CD44 mAb Hermes-1. Notice the absence of binding in the section treated with hyaluronidase (C) and in the section in which the assay was performed in the presence of Hermes-1 (E).
Immunohistochemistry
Immunohistochemical staining of endothelial structures was performed using primary antibodies anti-CD34, anti-CD31, or mouse immunoglobulin G1 (IgG1) isotype (all at 10 μg/mL in phosphate-buffered saline [PBS]/10% fetal bovine serum [FBS]), biotinylated secondary antibody, and streptavidin-horseradish peroxidase. Chromagen was NovaRed, and sections were counterstained with hematoxylin. Histochemical localization of HA was performed as described in Figure 2.6
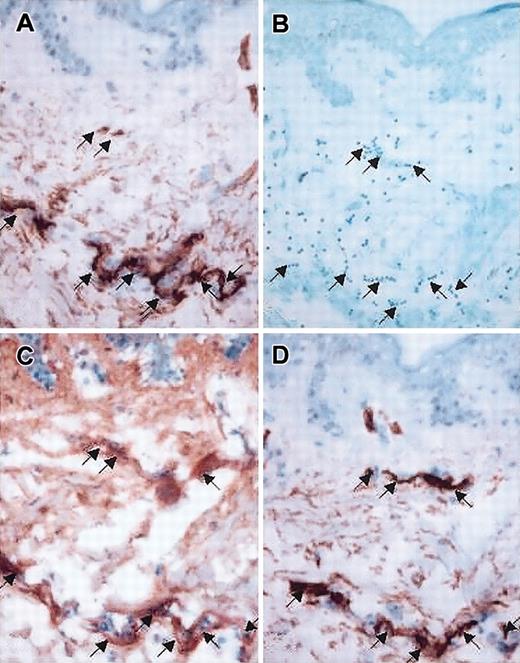
Photomicrographs of sequential sections of skin biopsy from a patient with acute cutaneous GVHD: colocalization of CD34 staining, HA deposition, and lymphocyte adherence. (A,D) Immunohistochemical stain for CD34 antigen, demonstrating location of endothelial structures (original magnification × 20). (B) Adherence assay result, showing lymphocytes (dark blue dots) attaching to dermal papillary structure (methyl green-thionin stain, original magnification × 20). (C) Distribution of HA (original magnification × 20), histochemically identified using a biotinylated bovine HA-binding proteoglycan (bPG); skin sections treated with hyaluronidase (20 U/mL) before incubation with bPG revealed no staining, and incubation of bPG with soluble HA (0.3 mg/mL) before treatment of skin sections also prevented staining, confirming the specificity of bPG for the detection of HA (data not shown). Note the intense staining of HA in defined structures of papillary/upper reticular dermis (C) correlating with staining for vascular endothelial antigen CD34 (A,D) and with the location of adherent lymphocytes (B) within these sequential sections (A-D, arrows).
Photomicrographs of sequential sections of skin biopsy from a patient with acute cutaneous GVHD: colocalization of CD34 staining, HA deposition, and lymphocyte adherence. (A,D) Immunohistochemical stain for CD34 antigen, demonstrating location of endothelial structures (original magnification × 20). (B) Adherence assay result, showing lymphocytes (dark blue dots) attaching to dermal papillary structure (methyl green-thionin stain, original magnification × 20). (C) Distribution of HA (original magnification × 20), histochemically identified using a biotinylated bovine HA-binding proteoglycan (bPG); skin sections treated with hyaluronidase (20 U/mL) before incubation with bPG revealed no staining, and incubation of bPG with soluble HA (0.3 mg/mL) before treatment of skin sections also prevented staining, confirming the specificity of bPG for the detection of HA (data not shown). Note the intense staining of HA in defined structures of papillary/upper reticular dermis (C) correlating with staining for vascular endothelial antigen CD34 (A,D) and with the location of adherent lymphocytes (B) within these sequential sections (A-D, arrows).
HA-binding assay
Adherence of lymphocytes and of KG1a cells4 to immobilized HA was performed in multiwell plates coated with HA (suspension dried onto plastic) from human umbilical cord and from rooster comb. Plates were treated with 3% bovine serum albumin (BSA) in RPMI 1640 for 2 hours at 37°C and were washed with cell adhesion medium (CAM) (RPMI 1640, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.2% bovine serum albumin [BSA], 1 mM Na pyruvate). Cells (106/mL in CAM) were centrifuged to contact the substrate, and binding was performed under static and shear (rotatory [80 rpm] and rocker [range, 5-30 cycles/min] platforms) conditions. Nonadherent cells were removed by vigorous washing with CAM. Bound cells were analyzed by light microscopy. Controls consisted of pretreatment of input lymphocytes with Hermes-1 mAb (10 μg/mL), treatment of HA-coated wells with hyaluronidase (20 U/mL), addition of exogenous soluble HA (0.1 mg/mL) to wells, and omission of HA in the wells.
Results and discussion
Endothelia of the papillary and upper reticular dermis in all aGVHD skin specimens supported lymphocyte adherence under Stamper-Woodruff assay conditions,1 but no binding was observed in normal skin specimens. The adherent lymphocytes appeared as clumps or chains (Figures 1B,2B), and their localization was traceable through consecutive sections of skin, indicating that binding was to specific structures. Neither chelation of divalent cations nor pretreatment of sections with neuraminidase, chymotrypsin, or bromelain had an effect on lymphocyte binding to skin endothelium of aGVHD, yet each abrogated the binding of lymphocytes to control lymph node high endothelial venules (HEVs). Thus, divalent-cation–dependent adhesion molecules such as platelet endothelial cell adhesion molecule-1 (PECAM-1), selectins, and integrins do not mediate binding, nor do sialoglycoproteins such as vascular adhesion protein-1 (VAP-1) and MECA-79 antigens.7,8
Treatment with heparitinase and keratanase had no effect on the binding of lymphocytes to endothelium, whereas hyaluronidase digestion of all aGVHD skin sections completely blocked lymphocyte adherence to endothelium (Figure 1). In contrast, hyaluronidase treatment of rat lymph node sections did not influence lymphocyte binding to HEV but completely removed HA (as measured by histochemistry) in lymph node and skin sections. Hence, abrogation of binding by hyaluronidase is a consequence of HA digestion and not a general inhibitory effect of enzyme treatment (or digestion conditions) on the capacity of endothelium to support lymphocyte adherence.
Because the principal lymphocyte membrane receptor for HA is CD44,9 we examined its role in binding lymphocytes to dermal endothelium in aGVHD. Hermes-15 completely inhibited lymphocyte binding to all aGVHD skin sections (Figure 1E), whereas isotype control mAb had no effect. Incubation of lymphocytes with Hermes-1 did not alter lymphocyte attachment to rat lymph node HEV, so the effect was not caused by a nonspecific alteration of lymphocyte-binding capabilities.
Representative results of histochemical staining of aGVHD skin are shown in Figure 2. Comparisons of the sites of adherent lymphocytes with endothelial structures and with the pattern of histochemical staining for HA revealed concordance: sites with intense HA staining (Figure 2C) corresponded with CD34 staining (Figure 2A,D), indicating that vascular endothelial structures (and not CD34–CD31+ lymphatic endothelium) laden with HA were the locations of lymphocyte adherence (Figure 2B). All skin specimens of aGVHD displayed intense endothelial staining for HA in at least some dermal vessels. In normal skin specimens, endothelial HA staining was prominent but typically less than that of aGVHD. Interestingly, within aGVHD specimens, no lymphocyte binding was observed on endothelial structures that lacked HA deposition. Although intense HA staining was observed at the dermalepidermal border and within glandular elements of the reticular dermis, lymphocytes did not adhere to these structures.
Under all conditions used (static, rotatory, or tilt platforms), there was no lymphocyte binding to human umbilical cord or rooster comb HA immobilized on plates, even at high concentrations. In contrast, cells of the hematopoietic cell line KG1a adhered readily to HA-coated plates under static and nonstatic conditions.10 KG1a adherence was CD44- and HA-specific and was abrogated by the Hermes-1 mAb, the treatment of HA-coated plates with hyaluronidase, and the addition of soluble HA.
Conspicuously, lymphocyte adherence to dermal vessels in aGVHD was not abrogated by soluble HA (up to 0.6 mg/mL), though input of 0.1 mg/mL was able to inhibit KG1a binding to HA-coated plates. Hence, the HA expressed at the dermal endothelium of aGVHD appears specialized to support lymphocyte adherence under shear force. The lack of lymphocyte adherence to immobilized HA is consistent with other results indicating that CD44 on resting lymphocytes does not readily engage HA and that activating cells during an allogeneic response in vivo11 or by exposing lymphocytes to anti-CD3 antibodies, phorbol esters, mitogens, inflammatory cytokines, or chemokines is required for HA binding.11-14 However, these other studies11-14 have only investigated lymphocyte binding to immobilized HA or to HA synthesized by endothelial cells in culture, not to HA in tissues in situ. Thus, our findings highlight an important difference between native, naturally expressed HA and HA presented on artificial solid supports or in cultures. Synthesis of HA is directed by HA synthases, which produce HA of different sizes.15 The affinity of CD44 for HA increases with the multivalency of HA.16 Thus, heterogeneity in size and lattice meshwork/microarchitecture17,18 may have significant functional consequences and may underlie the apparent difference observed here in the binding of lymphocytes to HA expressed by dermal endothelium and the absence of binding to epidermal HA or to chemically purified, immobilized HA.
HA is anchored to the vascular lumen by CD44 expressed on endothelial cells.19 HA deposits can mediate the “rolling phase” contacts of lymphocytes on the endothelial surface in vivo, and the role of CD44-HA interactions in directing lymphocyte trafficking to inflammatory sites is well recognized.12,20,21 Inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) up-regulate HA synthesis primarily by small vessel endothelial cells, including dermal microvascular cells.22 These same cytokines also up-regulate CD44 anchoring/binding of HA14,19 and have been linked pathobiologically to GVHD.23 Moreover, lymphocyte activation increases the capacity of CD44 on lymphocytes to engage surface HA.12,13 Altogether, these observations indicate that CD44-HA interactions are optimized to recruit lymphocytes at sites of inflammation. In fact, interrupting CD44-HA interactions significantly inhibits skin inflammatory responses such as contact hypersensitivity reactions.24
Our results provide the first evidence that deposits of HA within dermal papillary endothelium may promote lymphocyte migration to skin in aGVHD. Notably, our previous studies showed that steroids down-modulated the capacity of dermal endothelium to support lymphocyte adherence in Stamper-Woodruff assays, commensurate with resolution of aGVHD.2 Steroids markedly inhibit HA synthesis/expression by preventing the transcription of HA synthase mRNA.25 Thus, our current results suggest that down-modulating HA synthesis/expression contributes to the therapeutic effect of steroids.
Interfering with the trafficking of lymphocytes to target tissues represents an alternative strategy to immunosuppressives or to T-cell depletion for preventing/controlling aGVHD, and it may preserve graft-versus-leukemia (GVL) and other immune responses. Our findings suggest that disrupting HA synthesis/deposition or CD44-HA interactions in cutaneous aGVHD may provide this therapeutic benefit.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-05-1500.
Supported by National Institutes of Health/National Cancer Institute grant RO1 CA 84156 (R.S.), the Dana and Edward Slatkin Research Fund (R.S.), and Harvard Skin Disease Research Center Core Grant P30AR42689.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Benjamin D. Hopkins for superb technical assistance and our patients, alive and deceased, for their courage and inspiration.