Perez-Simon et al have recently reported an association between the development of extensive chronic graft-versus-host disease (cGVHD) and the dose of CD34+ cells infused, in patients undergoing allogeneic transplantation with reduced intensity conditioning.1 Furthermore, the increase in cGVHD seen with higher CD34+ cell doses is associated with lower disease relapse rates, and the authors conclude that the CD34+ content of the graft should be manipulated to prevent cGVHD in patients at low risk of relapse and to maximize disease control in those who are at high risk. This association is independent of the dose of CD3+ cells infused, and was described in a cohort of 86 patients with HLA-matched sibling donors receiving peripheral blood stem cells (PBSCs) mobilized by granulocyte colony-stimulating factor (G-CSF). This is in keeping with the previously observed correlation between higher CD34+ cell doses and the development of cGVHD in patients receiving unmanipulated PBSCs.2
We present 63 patients who have undergone transplantation with reduced intensity conditioning in this institution from June 1997 to June 2003, using fludarabine (30 mg/m2) from day –7 (D –7) to D –3, melphalan (140 mg/m2)D –2, and alemtuzumab (either 20 mg/d from D –8toD –4, or 30 mg/d D –8 and D –7). Of the 63 patients, 50 had HLA-matched sibling donors, and 13 had unrelated donors, of whom 6 were mismatched at up to 2 HLA class I or class II alleles. All the patients received G-CSF–mobilized PBSCs. Cyclosporin (3 mg/kg) was given intravenously from D –1 as prophylaxis for GVHD, and the GVHD occurrence described below relates to the period prior to any subsequent donor lymphocyte infusions (minimum 6 months after transplantation).
The median CD34+ dose was 5.3 × 106/kg (range 0.9-21.1 × 106/kg). Rates of grades II-IV acute GVHD and chronic GVHD were 12 (19%) of 63 and 12 (20%) of 59, respectively, for the entire patient group, with extensive chronic GVHD occurring in 9 (15%) of 59 patients. For sibling donors, the acute GVHD and cGVHD rates were 14% and 17% (extensive chronic GVHD, 13%), and for the unrelated donors, 38% and 33% (extensive chronic GVHD, 25%), respectively. This compares with 30% grades II-IV acute GVHD and 56% cGVHD (extensive cGVHD, 31%) in the sibling cohort reported by Perez-Simon et al.1
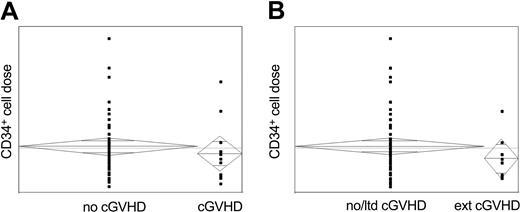
In our experience, no significant association could be observed between the occurrence of acute or chronic GVHD (limited or extensive) and the CD34+ dose infused for the cohort as a whole (Figure 1), or when subanalyzed by donor type, although this must be interpreted with caution as the numbers affected are small. Furthermore, no impact on event-free or overall survival could be demonstrated with increasing CD34+ cell dose, and similarly, no impact of CD34+ cell dose on the timing of onset or persistence of chronic GVHD was seen.
Correlation between CD34+ cell dose and the occurrence of chronic GVHD. All chronic GVHD, both limited and extensive. (B) Extensive chronic GVHD.
Correlation between CD34+ cell dose and the occurrence of chronic GVHD. All chronic GVHD, both limited and extensive. (B) Extensive chronic GVHD.
In addition, despite similar CD34+ doses, the occurrences of both acute and particularly chronic GVHD were markedly less than in the paper of Perez-Simon et al.1 This is presumed to reflect the effect of in vivo alemtuzumab, both in depleting T cells from the incoming graft and potentially in depleting antigen-presenting cells from the recipient.3-5
We would thus conclude that, in reduced intensity transplantation with in vivo T-cell depletion, increasing CD34+ dose is not associated with increased incidence of extensive chronic GVHD, and that manipulation of the CD34+ content of the graft may not result in therapeutic benefit.