Abstract
Patients given unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) for prophylaxis or treatment of thrombosis sometimes suffer serious bleeding. We showed previously that peptides containing 3 or more tandem repeats of heparin-binding consensus sequences have high affinity for LMWH and neutralize LMWH (enoxaparin) in vivo in rats and in vitro in citrate. We have now modified the (ARKKAAKA)n tandem repeat peptides by cyclization or by inclusion of hydrophobic tails or cysteines to promote multimerization. These peptides exhibit high-affinity binding to LMWH (dissociation constant [Kd], ≈ 50 nM), similar potencies in neutralizing anti–Factor Xa activity of UFH and enoxaparin added to normal plasma in vitro, and efficacy equivalent to or greater than protamine. Peptide (ARKKAAKA)3VLVLVLVL was most effective in all plasmas from enoxaparin-treated patients, and was 4- to 20-fold more effective than protamine. Several other peptide structures were effective in some patients' plasmas. All high-affinity peptides reversed inhibition of thrombin-induced clot formation by UFH. These peptides (1 mg/300 g rat) neutralized 1 U/mL anti–Factor Xa activity of enoxaparin in rats within 1 to 2 minutes. Direct blood pressure and heart rate measurements showed little or no hemodynamic effect. These heparin-binding peptides, singly or in combination, are potential candidates for clinical reversal of UFH and LMWH in humans.
Introduction
Treatment and prevention of thrombosis are major clinical concerns for medical and surgical patients throughout the world. Until recently, unfractionated heparin (UFH) and warfarin sodium have been the drugs of choice for treatment and prevention of thrombotic disorders. UFH also is used routinely to maintain adequate anticoagulation during coronary bypass surgery. However, each of these agents presents difficulties in management. For example, hospitalization is required for UFH infusion, and frequent laboratory monitoring is required for patients treated with warfarin to stabilize the level of anticoagulation. In the last several years, the low-molecular-weight heparins (LMWHs) have been used increasingly for treatment of deep vein thrombosis and for thromboprophylaxis in a wide variety of clinical situations, including prophylactic treatment of patients undergoing various types of orthopedic and abdominal surgery, patients with acute coronary syndrome, pregnant women with a predisposition to thrombotic disease leading to miscarriage, and cancer patients with a high risk of thrombotic events. LMWHs, unlike UFH, can be self-administered on an outpatient basis and require little monitoring compared with warfarin, and therefore have a potential economic advantage and are more convenient for the patients. All anticoagulants have the undesirable effect that 1% to 2% of the patients treated suffer significant and possibly life-threatening bleeding. Protamine is used routinely to neutralize UFH after coronary bypass and for patients who suffer bleeding while under treatment. However, protamine can induce serious side effects such as anaphylaxis or severe decreases in blood pressure and heart rate. Protamine is largely ineffective in patients who bleed while being treated with LMWH.1,2
We have now designed a class of peptides that, unlike protamine, is capable of neutralizing the LMWH enoxaparin in blood obtained from patients who are being treated with enoxaparin, is more efficient at neutralizing UFH in vitro than is protamine, and has little or no hemodynamic toxicity in rats compared with protamine. These peptides are concatamers of specific amino acid sequences that are common to a variety of heparin-binding proteins.3 We have described previously our work with the fundamental concatameric structures.4,5 We report here modifications of these peptides, and expect that these modified peptides will have greater clinical efficacy than those described in our previous studies.
Patients, materials, and methods
Human and animal experimentation
Healthy volunteers were recruited as approved by the institutional review board. Patients who were undergoing treatment with enoxaparin for thromboprophylaxis or antithrombotic therapy were recruited to the study by their physicians. Signed informed consent was obtained from healthy volunteers and patients through procedures approved by the institutional review board. Blood was collected by hospital phlebotomists. Animal protocols were approved by the Institutional Animal Care and Use Committee and the US Army Medical Research & Material Command, and were carried out under AAALAC-I guidelines. Sprague-Dawley male rats were purchased from Charles River Laboratories (Wilmington, MA) and used at 300 to 400 g.
Materials
Enoxaparin (Lovenox; Aventis, Bridgewater, NJ), protamine sulfate (Eli Lilly, Indianapolis, IN), the ATIII-binding heparin pentasaccharide fondaparinux (Arixtra; Sanofi, Malvern, PA), and porcine unfractionated heparin were obtained from our hospital pharmacy. Human α-thrombin was obtained from Enzyme Research Laboratories (South Bend, IN). Anti–Factor Xa assay kits (Stachrom Heparin) were purchased from Diagnostica Stago (Piscataway, NJ).
Preparation of peptides
Peptides were synthesized in the Protein Chemistry core facility at the Kimmel Cancer Center of this institution. Peptides were synthesized by standard solid phase synthesis using Fmoc (N-(9-fluorenyl)methyoxycarbonyl) chemistry. Peptide molecular weight was verified by mass spectrometry and amino acid content, and purity was verified by amino acid analysis and high-pressure liquid chromatography.
Affinity coelectrophoresis (ACE) experiments
Whole heparin from pig intestinal mucosa (Grade I-A; Sigma Chemical, St Louis, MO) was tyramine end-labeled and radiolabeled with 125Iodine (125I) to a specific activity about 1 × 107 cpm/μg. Radiolabeled heparin was passed over Sephadex G-100 (Sigma) and the final approximately 12% of the material, which represents the low–relative molecular mass (Mr) heparin of 6 kDa or less, were used in the binding assays.
Analytical ACE was carried out as described6 to characterize the affinities and selectivities of heparin-peptide interactions. In brief, peptides were dissolved in 2 × concentrated ACE running buffer and agarose minus the sodium acetate (1 × running buffer was 50 mM sodium MOPSO [3-(n-morpholino)-2-hydroxypropanesulfonic acid]/125 mM sodium acetate, pH 7.0) and poured into agarose wells. Heparin was then electrophoresed through the peptide-containing wells. ACE gels were then dried and heparin mobility was measured using a Phosphorimager (Molecular Dynamics, Piscataway, NJ) by scanning protein lanes and determining relative radioactivity per 88-μm pixel along the length of the lane. The dissociation constant (Kd) is calculated as the protein concentration at which the heparin is half shifted from being fully mobile at very low protein concentrations to being maximally retarded at saturating protein concentrations.
Effects of peptides on the reversal of anti–Factor Xa activity of UFH and enoxaparin in vitro
Peptides were tested as described4 either in the presence of 0.32% sodium citrate alone or in plasma anticoagulated with 0.32% sodium citrate. Anti–Factor Xa assays were performed using the anti–Factor Xa assay kit. The heparin/ATIII complex was allowed to form at 37°C for 2 minutes, peptide was added in concentrations of 1.2 to 120 μg/mL citrate or plasma, the mixture was incubated for 5 minutes, Factor Xa and finally the color reagent were added, and the absorbance at 405 nm was read on a spectrophotometer. The degree of neutralization was calculated as the units per milliliter of heparin neutralized by a given amount of peptide. The standard A405 curve for anti–Factor Xa activity of enoxaparin was generated based on the activity stated by the manufacturer, and this was used to calibrate the anti–Factor Xa activities of UFH and fondaparinux.
Effects of peptides on reversal of inhibition of thrombin activity by UFH and enoxaparin in vitro
Plasma was obtained from healthy donors. The assay was carried out in glass tubes as described previously.4 The thrombin concentration was standardized to produce a clotting time of 20 to 22 seconds. Heparin was added at 0.5 IU antithrombin activity/mL. The thrombin clotting time for heparin was approximately 3 minutes. In other experiments, enoxaparin was added at 0.5 U/mL anti–Factor Xa activity, consistent with plasma levels of enoxaparin expected in patients. The thrombin clotting time for enoxaparin was 48 seconds. To test the effects of the peptides, one minute after addition of heparin or enoxaparin to the plasma, the peptides were added in concentrations ranging from 1 to 200 μg/mL. After one minute, thrombin was added and the clotting time was determined by visual inspection.
Effects of peptides on platelet aggregation
Aggregation was performed on citrated platelet-rich plasma containing 0.5 U/mL enoxaparin on a Chrono-Log aggregometer (Chronolog, Havertown, PA). Peptide concentration in all experiments was 12 μg/mL. Agents used were 2 to 8 μM adenosine diphosphate (ADP; Sigma), 0.9 to 1.5 mg/mL ristocetin (Sigma), 5 to 25 μM calcium ionophore A23187, 2 to 8 μM epinephrine (Chronolog), and thrombin receptor agonist peptide (TRAP) peptide (Bachem, King of Prussia, PA).
Effects of peptides on reversal of enoxaparin anti–Factor Xa activity in vivo in rats
Rats were anesthetized by inhalation of 1% isoflurane. The left jugular vein and right femoral vein were cannulated. Enoxaparin and peptides were infused through the jugular vein and blood was collected from the femoral vein. Blood samples were all 0.1 mL. Blood was immediately transferred to tubes containing 3.2% citrate to obtain a final concentration of 0.32% citrate. Blood was drawn immediately before infusion of enoxaparin to establish baseline anti–Factor Xa activity. Enoxaparin (43 IU anti–Factor Xa activity/kg in 0.1 mL phosphate-buffered saline) was infused, followed immediately by 0.2 mL saline to ensure complete delivery of the anticoagulant. At this dosage, the plasma anti–Factor Xa activity was maximal at about 1 U/mL 3 minutes after injection. Blood was collected every 30 seconds for 3 minutes. The peptides were infused 3 minutes after enoxaparin infusion, followed by a 0.2 mL saline flush, and blood collection was resumed every 30 seconds until 10 minutes after enoxaparin infusion, and at 15, 20, 25, and 30 minutes. The animals were killed by intracardiac injection of 0.2 mL Beuthanasia D. The blood samples were centrifuged to obtain plasma and were assayed for anti–Factor Xa activity as described above.
Effects of peptides on hemodynamic functions in rats
The animals were anesthetized by inhalation of 1% isoflurane. Hemodynamic measurements were made with the Direct Blood Pressure Monitoring System (Kent Scientific, Torrington, CT) and data were collected into a computer and analyzed by the Dasylabs program (Dasytec, Amherst, NH). The left femoral artery was cannulated for blood pressure and heart rate measurements, the oxygen saturation was measured by a pulse oximeter, and respiration was measured via a cuff around the chest. The procedure was as follows. First, saline was injected and the animal was monitored for hemodynamic stability. Then enoxaparin was infused, followed 3 minutes later by infusion of either protamine or the test peptide at up to 2 mg peptide per rat. The animal was monitored for an additional 25 minutes. Peptide effects on hemodynamic parameters were also monitored without prior injection of enoxaparin. In a number of experiments, peptides that had caused no adverse hemodynamic parameters in several rats were injected into other rats, followed after 10 minutes by injection of protamine, and the animals were monitored for an additional 20 minutes. Animals were killed at the end of these procedures by intracardiac injection of 0.2 mL Beuthanasia D.
Results
Affinity constants for peptide low-Mr heparin binding
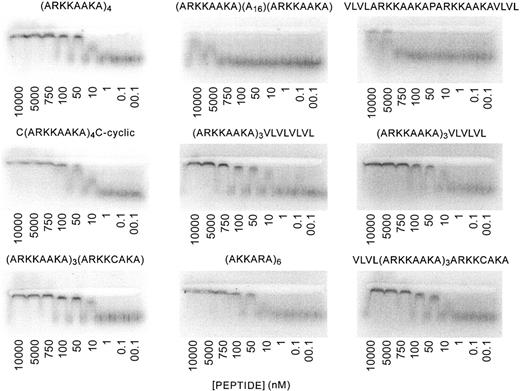
ACE gels were run with the low-Mr fraction of 125I UFH against the various peptides as described previously.5 The binding constants are shown in Table 1. Peptides with high affinity for heparin were (ARKKAAKA)4, C(ARKKAAKA)3C (cyclized), (ARKKAAKA)3ARKKCAKA, (ARKKAAKA)3VLVLVLVL, (AKKARA)6, (ARKKAAKA)3VLVLVL, and VLVL(ARKKAAKA)3ARKKCAKA. The Kds were similar to each other (≈ 50 nM). In contrast, the peptides ARKKAAKA(A16)ARKKAAKA and VLVLARKKAAKAPARKKAAKAVLVL had very low affinity for heparin. Representative ACE gels are shown in Figure 1.
ACE analysis of binding of peptides to low-molecularweight heparin. ACE6 was used to study interactions between peptides and low-Mr heparin. Trace concentrations of radiolabeled heparin are electrophoresed through agarose lanes containing proteins at various concentrations. The electrophoretic patterns of radiolabeled heparin are then visualized using a Phosphorimager, and Kds of peptide-heparin interactions were calculated from binding plots. Per peptide, 4 assays were performed. Results of all the experiments are shown in Table 1. The panels show the Phosphorimager tracings of the ACE gels, and the concentration of peptide used in each lane is shown.
ACE analysis of binding of peptides to low-molecularweight heparin. ACE6 was used to study interactions between peptides and low-Mr heparin. Trace concentrations of radiolabeled heparin are electrophoresed through agarose lanes containing proteins at various concentrations. The electrophoretic patterns of radiolabeled heparin are then visualized using a Phosphorimager, and Kds of peptide-heparin interactions were calculated from binding plots. Per peptide, 4 assays were performed. Results of all the experiments are shown in Table 1. The panels show the Phosphorimager tracings of the ACE gels, and the concentration of peptide used in each lane is shown.
Neutralization of the anti–Factor Xa activity of unfractionated heparin by peptides in vitro
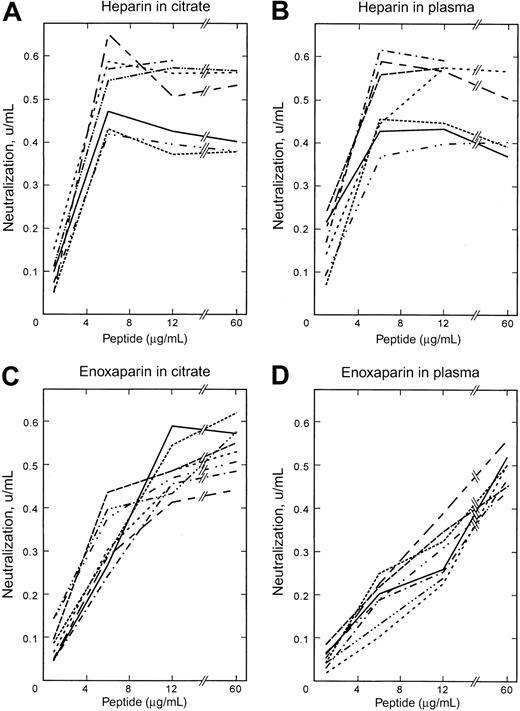
Figure 2A-B shows the ability of the peptides and protamine to neutralize the anti–Factor Xa activity of UFH in the presence of either citrate alone or when added to normal citrated plasma. The peptides with very weak affinity for heparin, that is, ARKKAAKA, ARKKAAKA(A16)ARKKAAKA, and VLVLARKKAAKAPARKKAAKAVLVL, had no effect on any of the heparin activities (not shown). All high-affinity peptides based on the ARKKAAKA motif as well as the peptide (AKKARA)6 were able to neutralize UFH as well as or better than protamine in the presence of plasma. With all peptides and for protamine, neutralization was maximal at 6 μg peptide/mL plasma. Peptides at this concentration neutralized about 0.55 to 0.6 U/mL of the 0.7 U/mL anti–Factor Xa activity that had been added to the plasma of UFH, while protamine neutralized only about 0.4 U/mL. Increasing the protamine concentration above 6 μg/mL in fact reduced its ability to neutralize anti–Factor Xa activity of UFH. The peptides with hydrophobic moieties (ARKKAAKA)3VLVLVLVL, (ARKKAAKA)3VLVLVL, and VLVL(ARKKAAKA)3ARKKCAKA were the most effective at neutralizing the heparin activity in normal plasma. The results represent the mean of triplicate assays at each peptide concentration from each of 3 volunteers. Reproducibility was within 10% for the 3 volunteers.
Effects of peptides on anti–Factor Xa activities in citrate and in plasma. The effect of peptides and protamine on neutralization of enoxaparin and unfractionated heparin was evaluated using the anti–Factor Xa assay kit in purified coagulation protein assays (ie, in sodium citrate) and in normal plasma. The X-axis indicates the concentration of peptide (μg/mL) relative to the volume of citrate or plasma used for the assay, and the Y-axis shows the amount of anti–Factor Xa activity neutralized (U/mL). (A) UFH in citrate. (B) UFH in plasma. (C) Enoxaparin in citrate. (D) Enoxaparin in plasma. Each point is the mean of triplicate analyses at each concentration from each of 3 healthy donors. Symbols represent peptides as follows: protamine,  ; (ARKKAAKA)4, — · · —; C(ARKKAAKA)4C, – – – – – – –; (ARKKAAKA)3ARKKCAKA, - - - - - - - - - - -; (ARKKAAKA)3VLVLVLVL,
; (ARKKAAKA)4, — · · —; C(ARKKAAKA)4C, – – – – – – –; (ARKKAAKA)3ARKKCAKA, - - - - - - - - - - -; (ARKKAAKA)3VLVLVLVL,  ; (AKKARA)6, — · · · — · · ·; (ARKKAAKA)3(VL)3, — · — · —; and VLVL(ARKKAAKA)3ARKKCAKA, — — — — — —.
; (AKKARA)6, — · · · — · · ·; (ARKKAAKA)3(VL)3, — · — · —; and VLVL(ARKKAAKA)3ARKKCAKA, — — — — — —.
Effects of peptides on anti–Factor Xa activities in citrate and in plasma. The effect of peptides and protamine on neutralization of enoxaparin and unfractionated heparin was evaluated using the anti–Factor Xa assay kit in purified coagulation protein assays (ie, in sodium citrate) and in normal plasma. The X-axis indicates the concentration of peptide (μg/mL) relative to the volume of citrate or plasma used for the assay, and the Y-axis shows the amount of anti–Factor Xa activity neutralized (U/mL). (A) UFH in citrate. (B) UFH in plasma. (C) Enoxaparin in citrate. (D) Enoxaparin in plasma. Each point is the mean of triplicate analyses at each concentration from each of 3 healthy donors. Symbols represent peptides as follows: protamine,  ; (ARKKAAKA)4, — · · —; C(ARKKAAKA)4C, – – – – – – –; (ARKKAAKA)3ARKKCAKA, - - - - - - - - - - -; (ARKKAAKA)3VLVLVLVL,
; (ARKKAAKA)4, — · · —; C(ARKKAAKA)4C, – – – – – – –; (ARKKAAKA)3ARKKCAKA, - - - - - - - - - - -; (ARKKAAKA)3VLVLVLVL,  ; (AKKARA)6, — · · · — · · ·; (ARKKAAKA)3(VL)3, — · — · —; and VLVL(ARKKAAKA)3ARKKCAKA, — — — — — —.
; (AKKARA)6, — · · · — · · ·; (ARKKAAKA)3(VL)3, — · — · —; and VLVL(ARKKAAKA)3ARKKCAKA, — — — — — —.
Neutralization of the anti–Factor Xa activity of enoxaparin by peptides in vitro in normal plasma
Figure 2C-D shows the neutralization of the anti–Factor Xa activity of enoxaparin added to citrate or to normal plasma. The same plasmas used for the UFH studies above were used for the enoxaparin studies. The low-affinity peptides ARKKAAKA, ARKKAAKA(A16)ARKKAAKA, and VLVLARKKAAKAPARKKAAKAVLVL had minimal effect on the anti–Factor Xa activity of enoxaparin (not shown). In citrate, protamine was more effective than the peptides for neutralization of enoxaparin. The apparent anti-FXa activities of protamine and all the peptides were considerably higher in citrate than in plasma. However, in plasma the peptides neutralized at least as much and in most cases more enoxaparin than did protamine. Neutralization by protamine and the high-affinity peptides was maximal at 12 μg/mL plasma. (ARKKAAKA)4, (ARKKAAKA)3ARKKCAKA, (ARKKAAKA)3VLVLVLVL, and VLVL(ARKKAAKA)3ARKKCAKA had similar effects to each other and all were more effective than protamine in the presence of plasma. (ARKKAAKA)3VLVLVL, C(ARKKAAKA)4C-cyclic peptide, and (AKKARA)6 were equivalent to protamine at 12 μg/mL.
Neutralization of anti–Factor Xa activity of heparin and enoxaparin by peptides in vitro in plasma from patients
Table 2 shows the neutralization of heparin in plasmas from 3 patients who were undergoing continuous heparin infusion at the time of blood collection. Protamine was able to reverse most of the activity in all 3 plasmas. The peptide (ARKKAAKA)3VLVLVLVL had activity equal to that of protamine in 2 of the 3 plasmas (patients 2H and 3H), and 67% of the activity of protamine in the plasma of patient 1H. The peptide (ARKKAAKA)3ARKKCAKA reversed 100% of the activity in patient 1H.
Table 3 shows the neutralization of enoxaparin in plasma obtained from patients who were treated with enoxaparin for their health conditions. The anti–Factor Xa levels at the time of blood collection ranged from 0.34 to 0.85 U/mL for the patients shown. The results for individual patients are shown in order to demonstrate the variability of response among individual patients. The results differ in several respects from the experiments shown in Figure 2C-D, in which enoxaparin was added to normal plasma. The peptide (ARKKAAKA)3VLVLVLVL was overall the most effective in the patients' plasmas, as it was in the normal plasma. In 6 of 9 patients, this peptide gave the highest degree of neutralization. In 2 of 9 patients' plasmas, VLVL(ARKKAAKA)3ARKKCAKA was the most effective, about 30% greater than (ARKKAAKA)3VLVLVLVL. The peptides (ARKKAAKA)3 VLVLVLVL and C(ARKKAAKA)4C were able to neutralize about 85% of the average amount neutralized in control enoxaparin-supplemented plasma, but the other peptides were able to neutralize on average about half the anti–Factor Xa activity in patients' plasma compared with normal plasma. There was considerable variability in the efficacy of the other peptides from patient to patient. Relative to the anti–Factor Xa levels at the time of blood collection, 12 μg/mL (ARKKAAKA)3VLVLVLVL was able to neutralize 53 ± 8% (range, 41%-74%) of the anti-FXa activity present, compared with 3.6 ± 3.5% neutralization by protamine. The range of actual amounts neutralized in the patients' plasma was 0.21 to 0.48 U/mL.
The peptides did not alter significantly the anti–Factor Xa levels in the plasmas of 2 enoxaparin-treated patients whose anti–Factor Xa activity at the time of blood collection was 0.2 U/mL or less.
Effects of peptides on fondaparinux
None of the peptides tested had significant ability to neutralize the anti–Factor Xa activity of the synthetic pentasaccharide fondaparinux under the assay conditions used in this study.
Effects of peptides on reversal of the effects of UFH and enoxaparin on thrombin clotting time
The high-affinity peptides (ARKKAAKA)4, C(ARKKAAKA)4C, (ARKKAAKA)3ARKKCAKA, (ARKKAAKA)3VLVLVLVL, and (AKKARA)6 all neutralized the UFH effect on thrombin at concentrations of 4 to 5 μg/mL. The same peptides neutralized the enoxaparin effect at, respectively, 17.5, 20, 15, 7.5, and 17.5 μg/mL. Thus the peptide (ARKKAAKA)3VLVLVLVL appeared to have the greatest effect on reversing the activity of enoxaparin in this assay.
Effects of peptides on platelet aggregation
Platelet aggregation was tested in blood from 3 healthy volunteers. No significant differences from controls, that is, aggregation in the absence of enoxaparin or peptides, were observed, with the exception of a modest change of the shape of the aggregation curve in response to collagen with peptide (ARKKAAKA)4 in one volunteer. There was no change in the aggregation pattern with the other agents tested in the presence of enoxaparin plus peptide, or with enoxaparin alone. Aggregation was not studied in patients' blood because of the difficulty in obtaining adequate volumes of blood for these experiments.
In vivo neutralization of enoxaparin in rats
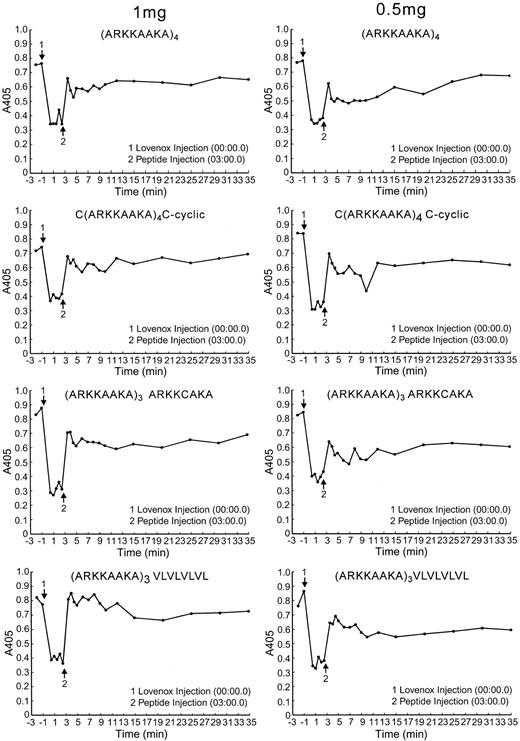
Figure 3 shows representative curves for neutralization of enoxaparin in rats. The peptides were injected 3 minutes after enoxaparin, when peak levels of enoxaparin of about 1 U/mL were achieved. All the high-affinity peptides administered at 2 mg/300 g rat (6.4 mg/kg) completely reversed anti–Factor Xa activity within a minute after injection (not shown). At least 2 rats were used for this peptide concentration. The peptide that was most dose-effective was (ARKKAAKA)3VLVLVLVL, which gave the greatest neutralization at 1 and 0.5 mg/kg (3.2 and 1.6 mg/300 g rat) (Figure 3). These data were derived from either 1 or 2 rats at each concentration for each peptide.
Effect of peptides on neutralization of enoxaparin in vivo in rats. Rats were cannulated in the jugular and femoral veins. Enoxaparin was injected through the jugular vein, and 3 minutes later the peptide was injected via the jugular vein. Blood was collected from the femoral vein; 0.1 mL was collected at each time point. Plasma was prepared from each tube, and the anti–Factor Xa assay was performed. The zero time point represents anti–Factor Xa activity in the assay before administration of enoxaparin. The A450 is inversely proportional to the enoxaparin concentration. A450 of 0.4 is equivalent to 1 U/mL anti–Factor Xa activity. The events at the arrows are indicated in the box insert. The data shown are limited to those 4 peptides that have been tested at both the 1 mg and 0.5 mg/300 g dosages.
Effect of peptides on neutralization of enoxaparin in vivo in rats. Rats were cannulated in the jugular and femoral veins. Enoxaparin was injected through the jugular vein, and 3 minutes later the peptide was injected via the jugular vein. Blood was collected from the femoral vein; 0.1 mL was collected at each time point. Plasma was prepared from each tube, and the anti–Factor Xa assay was performed. The zero time point represents anti–Factor Xa activity in the assay before administration of enoxaparin. The A450 is inversely proportional to the enoxaparin concentration. A450 of 0.4 is equivalent to 1 U/mL anti–Factor Xa activity. The events at the arrows are indicated in the box insert. The data shown are limited to those 4 peptides that have been tested at both the 1 mg and 0.5 mg/300 g dosages.
It is important to note the differences in routes of administration of enoxaparin to rats that we have used here compared with standard human treatment. The LMWHs are all administered subcutaneously in human studies, and plasma anti–Factor Xa activity is maximal 4 hours after the injection. We attempted to administer enoxaparin subcutaneously to follow the convention for human use. However, we could not detect anti–Factor Xa activity in the blood over periods of 1 to 4 hours after subcutaneous injection of enoxaparin at several sites in the rats (left and right flanks and between shoulder blades). Likewise, several attempts at intramuscular injections did not give measurable anti–Factor Xa activity. Therefore we were restricted to the use of the intravenous route.
Effects of peptides on blood pressure and heart rate
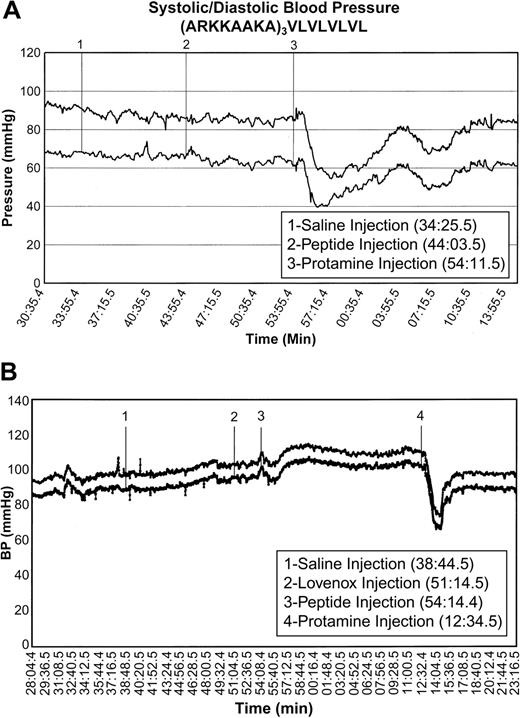
In the presence of enoxaparin, 12 of 16 rats tested with protamine, and 3 tested with protamine alone, suffered a severe blood pressure drop of at least 30 mmHg in both systolic and diastolic pressures, which lasted 10 minutes, accompanied by a parallel drop and subsequent recovery in heart rate; 1 had a milder response (a drop of 20 mm for 5 minutes), and 3 had no response. In contrast to this deleterious effect of protamine, both in the presence and absence of enoxaparin the peptides showed little or no toxicity in terms of their effect on blood pressure, heart rate, respiration rate, and oxygen saturation. When a blood pressure decrease occurred, it was in the range of 10 to 30 mmHg but never lasted for longer than 2 minutes. The very low-affinity peptide ARKKAAKA(A16)ARKKAAKA did not alter the blood pressure. In general, animals that had no adverse hemodynamic response to peptide and that were subsequently administered protamine then showed a response to protamine similar to that of animals that had received protamine alone. However, in a number of cases the peptide-treated animals showed no response to the subsequent protamine injection, but the results were not consistent with any given peptide. Typical results are shown in Figure 4 and the overall results are summarized here: (ARKKAAKA)4, 6 of 8 rats showed no response and 2 of 8 had a mild response; C(ARKKAAKA)4C-cyclized, 6 of 6 rats showed no response; (ARKKAAKA)3ARKKCAKA, 6 of 7 rats showed no response to peptide; and (ARKKAAKA)3VLVLVLVL, 2 of 7 rats showed a delayed drop of 30 and 45 mm at about 3 minutes and again at 6 minutes after peptide infusion, each time with a quick recovery within 1 minute. The others had no changes in blood pressure. Peptides with variations of this structure, (ARKKAAKA)3(VL)6 and (ARKKAAKA)4VLVLVLVL, each injected into 2 animals, did not affect the hemodynamic functions. However, (ARKKAAKA)3VLVLVLcaused hypotension in 2 of 2 rats tested similar to that induced by protamine.
Effects of peptides on blood pressure and heart rate in rats. Rats were sedated and catheterized for administration of drugs and measurement of hemodynamic parameters as described in the text. At the indicated times the following actions were taken: administration of saline to ensure that the animal and intubations were stable; administration of enoxaparin; administration of test peptide (or protamine alone); and administration of protamine following nonresponse to peptide. Systolic and diastolic blood pressure are shown. This pair of graphs demonstrates the absence of blood pressure changes after administration of 1 mg/300 g rat of (ARKKAAKA)3VLVLVLVL in the presence and absence of enoxaparin. Other animals given this peptide had been observed to have stable blood pressure when monitored for 30 minutes with no subsequent protamine administration. Upper curve: systolic pressure. Lower curve: diastolic pressure. The results with animals treated with each peptide are summarized in the text.
Effects of peptides on blood pressure and heart rate in rats. Rats were sedated and catheterized for administration of drugs and measurement of hemodynamic parameters as described in the text. At the indicated times the following actions were taken: administration of saline to ensure that the animal and intubations were stable; administration of enoxaparin; administration of test peptide (or protamine alone); and administration of protamine following nonresponse to peptide. Systolic and diastolic blood pressure are shown. This pair of graphs demonstrates the absence of blood pressure changes after administration of 1 mg/300 g rat of (ARKKAAKA)3VLVLVLVL in the presence and absence of enoxaparin. Other animals given this peptide had been observed to have stable blood pressure when monitored for 30 minutes with no subsequent protamine administration. Upper curve: systolic pressure. Lower curve: diastolic pressure. The results with animals treated with each peptide are summarized in the text.
Discussion
We have previously described the high affinity for heparin of the (ARKKAAKA)n tandem repeat peptides and their ability to neutralize activities of UFH and enoxaparin.4,5 The goal of our current study was to modify the structure of these peptides in various ways to enhance heparin-neutralizing activity in vivo. The first strategy was to promote peptide multimerization by modifications at or near peptide termini. These modifications included hydrophobic tails to promote hydrophobic interactions and single cysteine residues to promote disulfide bonding. Another approach was to cyclize the peptide, which is a modification that often dramatically enhances the biologic activities of various peptides.
This approach has generated peptides that are potentially useful antidotes for patients who bleed while being treated with LMWHs, in this particular case enoxaparin, or with UFH. The peptides efficiently neutralize both the antithrombin and anti–Factor Xa activities of UFH and the anti–Factor Xa activity of enoxaparin. We have found highly significant differences between the effects of the peptides on anti–Factor Xa assays in normal plasma to which enoxaparin is added in comparison with plasma from patients undergoing treatment with enoxaparin. In agreement with studies by others who tested native LMWHs in vitro,1,2,7,8 we found that protamine was able to neutralize somewhat less than half the anti–Factor Xa activity of enoxaparin in vitro. Our high-affinity heparin-binding peptides, especially those with hydrophobic regions, are somewhat better than protamine in neutralizing enoxaparin added to normal plasma. However, in contrast, protamine had little effect in the plasmas from patients who had been treated with enoxaparin. Our protamine data are consistent with the known lack of efficacy of protamine for treatment of patients who bleed when treated with LMWH. Surprisingly, we encountered substantial variability in the efficacy of our high-affinity peptides in the plasmas from patients. The peptide (ARKKAAKA)3VLVLVLVL was the most consistently effective at neutralizing enoxaparin in plasma from enoxaparin-treated patients. This peptide was from 4 to 20 times more effective than protamine when added to patient plasma. Neutralization of anti–Factor Xa activity in patient plasma by this peptide was equivalent to that in normal plasma containing unmetabolized enoxaparin. Other structures that were effective in patients' plasmas, but with substantial individual variation, were the C(ARKKAAKA)C-cyclic peptide and the peptide that contained a short hydrophobic sequence and an internal cysteine, VLVL(ARKKAAKA)3ARKKCAKA.
The mechanism by which the interactions of peptides with enoxaparin differ when enoxaparin is added to normal plasma or assayed in plasma from patients is not known. This finding was unexpected, since all the peptides exhibited similar Kds for binding to low-Mr heparin. In addition, the published Kd for protamine binding to heparin9 is similar to that of our peptides. From this and our previous study,5 it might be concluded that including 3 or more repeats of the basic (ARKKAAKA) sequence in a peptide confers a maximal binding affinity for LMWH and minor manipulations of peptide structure beyond this cannot enhance binding affinity further. Thus we hypothesize that specific structural features of the peptides in addition to those that determine affinity (Kd) for heparin must be involved in binding to the specific structures of the heparin fragments containing the anticoagulant activity. Although the time course of appearance and disappearance of anti–Factor Xa activity in the plasma after enoxaparin treatment is well documented,10,11 there are no published studies describing how the heparin molecules are altered during circulation in the blood. We suggest that enoxaparin is metabolized in the circulation of the patients to shorter fragments that can no longer bind to protamine, yet retain the ability to bind to, and be neutralized by, several of our peptide structures. An alternative explanation should be considered based on the study of Crowther et al.12 That study analyzed native LMWH preparations and concluded that the degree of sulfation of the different preparations of LMWH, rather than the chain length, correlated best with the ability to bind to protamine. Desulfation in vivo, or specific elimination of chains with specific sulfation patterns, although not yet demonstrated, could be another mechanism by which the ability of protamine, and indeed some of our peptide structures, to neutralize LMWH in vivo is reduced. If our assumptions about the importance of the variations of both peptide structure and in vivo metabolism of enoxaparin are correct, then the optimal LMWH reversal agent may consist of mixtures of 2 or more specific peptide structures to take into account the differences in metabolism of LMWH between patients or as a function of time elapsed following the injection of LMWH.
With regard to UFH, our peptides are as effective as or better than protamine for neutralization of the anti–Factor Xa activity both when UFH is added to normal plasma and when plasma is obtained from patients who have been infused with UFH. Since the patients were undergoing a continuous infusion with UFH at the time their blood was collected, metabolism of UFH would not be apparent in our samples to the degree that we observed in the plasmas of enoxaparin-treated patients.
It is of interest to consider peptide designs that were not effective in meeting our goals. The peptide VLVLARKKAAKAPARKKAAKAVLVL was designed to assume a hairpin-like conformation that could bind LMWH fragments with high affinity between the peptide's 2 basic arms. A central proline was included to form a hinge, and the placement of hydrophobic tails at each end were intended to promote intrapeptide associations once the heparin-fragment was bound. Another design, ARKKAAKA(A16)ARKKAAKA, was used to test the importance of spacing of contiguous arrangement of consensus sequences. Both peptides exhibited very weak affinity for heparin and insignificant activity in our heparin-neutralization assays. These findings suggest the importance of the contiguous arrangement of heparin-binding consensus sequences. We have previously observed that inclusion of one or more prolines in other peptide constructs were disruptive to heparin-binding.5
Neutralization of the antithrombin and anti–Factor Xa effects of UFH by protamine and all the peptides in vitro was maximal at about 6 μg/mL, a clinically relevant plasma concentration. Higher concentrations of protamine or peptides generally led to an apparent decrease in neutralization. Neutralization of the anti–Factor Xa activity of enoxaparin was maximal at 12 μg/mL for all of the high-affinity peptides. In contrast to the reversal of activity with UFH, there was a modest but consistent increase in neutralization at much higher peptide concentrations.
There are 3 other laboratories that have designed peptides that have been proposed as effective antiheparin agents.13-15 The Wakefield peptide13 [+18RGD] contains pairs of arginine interspersed between 1 or 2 alanines. These sequences are very different from the Cardin and Weintraub3 consensus sequences. The RGD sequence is a strong cell-binding domain. The peptide of Shenoy et al (HepArrest)14 is a complex structure in which 3 13–amino acid chains are tethered to a common backbone. The peptide of Chang et al15,16 and Lee et al17 is a 15–amino acid arginine-rich proteolytic fragment of protamine called low-molecular-weight protamine (LMWP). The HepArrest peptides are engineered to have intrinsic partial alpha-helical structure, which would presumably cause the bound heparin to assume an alpha-helical structure. Our peptides are not intrinsically alpha-helical, but assume an alpha-helical conformation when bound to heparin.5 Thus, our peptides may have more flexibility to conform to a variety of heparin sequences encountered in any of the therapeutic heparin formulations or their metabolites.
Our studies have shown that our high-affinity heparin-binding peptides neutralize 1 U/mL enoxaparin anti–Factor Xa activity in rats within a minute. Hulin et al have reported efficacy of their [+18RGD] peptide for neutralization of the LMWH dalteparin sodium (Fragmin; Pharmacia, Bridgewater, NJ) in dogs.18 The LMWP fragment can neutralize UFH in dogs, but has not been tested with LMWH.15-17 It is difficult to compare these data with ours because peptides were administered at different times after LMWH, the actual levels of anti–Factor Xa activity at the time of the peptide infusion are not described, and only the percentage, but not the actual amounts, of heparin neutralized, are given. The concentration of the LMWP fragment required to neutralize different types of heparin in vitro was several times higher than that of the native protamine,17 suggesting that very high dosages may be needed for therapeutic effect.
A major problem with protamine is hemodynamic toxicity. Our in vivo studies with rats suggest that our peptides have little or no hemodynamic toxicity, whereas the same amount of protamine caused a severe and sustained drop in blood pressure and heart rate in most of the rats that we studied. Oxygen saturation and respiration were unaffected by the peptides. The peptides of Hulin et al had low hemodynamic toxicity in dogs.18 The peptides of Shenoy et al14 induced an increase in blood pressure, and the LMWP of Lee et al17 caused a significant drop in blood pressure but less than the parent protamine. Our peptides may have an advantage in terms of hemodynamic toxicity. This may be due in part to the mixture of R and K basic residues or the spacing of the basic residues within the peptides in comparison with other published basic peptide sequences.
Another concern with the use of peptides as therapeutic agents is toxicity via their effect on physiologic activities in the patient. A recent study has shown that 2 of the peptides that we have designed do not affect the activity of mast cell proteases.19
Alternatives to UFH and LMWH for treatment and prophylaxis of thrombosis are under active development. These include further modifications and refinements of heparins. The synthetic pentasaccharide fondaparinux20 comprises the exact ATIII heparin-binding sequence. No reversal agent is available for this anticoagulant. The peptides reported in this study do not appear to neutralize fondaparinux. A new method for processing UFH to give relatively homogeneous LMWHs that are designed rationally to maximize antithrombin or anti–Factor Xa activity21 can be neutralized by protamine in vivo and therefore is likely to be reversible by the peptides described in this paper. No reversal agents are available for the non–heparin-based oral anticoagulant ximelagatran (Astra-Zeneca, Waltham, MA). All these anticoagulants induce similar incidence of serious bleeding. Thus the availability of a strong neutralizing agent with minimal or no hemodynamic toxicity potentially could allow a means to provide patients with safer anticoagulation.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-07-2334.
Supported by Subagreement 2000-79-TJUniversity-SCHICK from the National Medical Technology Testbed (NMTB) under US Army Medical Research Acquisition Activity Cooperative Agreement no. DAMD17-97-2-7016.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jose Martinez for helpful advice. We thank Lynda Thomson and the Jefferson Antithrombotic Service for their assistance. We thank Andrew Likens for preparation of the illustrations.
The content of the information does not necessarily reflect the position or the policy of the NMTB, and no official endorsement should be inferred.