Abstract
A 4-base deletion has been identified in the coding region of the gene for gastric intrinsic factor (IF) in an 11-year-old girl with severe anemia and cobalamin (Cbl) deficiency. The bone marrow showed frank megaloblastic morphology, and the Schilling test indicated a failure to absorb Cbl that was corrected by coadministration of IF. Pentagastrin administration induced acid secretion, but the gastric juice lacked IF as determined by CbI binding, by fractionation of protein-bound CbI, and by immunoprecipitation with anti-IF antiserum. Individual exons were amplified by the polymerase chain reaction by using primers to the flanking intronic regions, and the nucleotide sequence analysis identified a 4-base deletion (c183_186delGAAT) spanning positions 104 to 107 in exon 2, resulting in premature termination of translation. This mutation also eliminates a site for Bst XI endonuclease and introduces a site for BsaBI for identifying this deletion in hereditary IF deficiency.
Introduction
Malabsorption of cobalamin (Cbl) can result from a number of causes such as acquired abnormalities of the stomach,1 in a familial disorder involving translocation of receptor-bound IF-Cbl,2 and deficiency of transcobalamin II (TCII), because this protein transfers the absorbed Cbl into the blood.3 The most common cause of Cbl malabsorption is pernicious anemia (PA) with atrophic gastritis, resulting in achlorhydria and reduced IF secretion.4 Patients have been described with juvenile PA,5 childhood familial malabsorption of Cbl,6 and juvenile congenital PA.7 The cloning of the gene for IF and localization to chromosome 11q138,9 provide the molecular probes to identify defects involving the IF gene.
Inherited IF deficiency is a rare form of Cbl malabsorption in which the gastric mucosa as well as acid and pepsin secretion is normal, but selective malabsorption of Cbl occurs because of lack of functional IF protein.10 The disorder presents in early childhood, and these patients invariably lack autoantibodies to IF or parietal cells.11 Many variants of IF deficiency have been described, including an unstable IF protein that is susceptible to acid and trypsin,12 an IF with lower affinity for Cbl and the ileal receptor,13 and an IF protein that binds Cbl but does not bind to the receptor.14 The genetic basis for the lack of a functional IF has not been defined in these patients.
Study design
Case report
The proband was the first child of a 15-year-old mother of African-American origin. The history provided by the mother indicated that the father was 18 years old and of the same racial origin. The child was initially evaluated for severe anemia at age 3 years. Bone marrow showed megaloblastic erythroid hyperplasia. On admission, she received packed red blood cells and was treated with 100 μg B12 injections weekly. At age 11, the patient was admitted to the hospital for recurrence of anemia because of discontinuation of therapy. She was treated with weekly vitamin B12 injections (1000 μg), and her hemoglobin increased to 13.3 g/dL (133 g/L). The hematologic findings from both admissions are summarized in Table 1. A Schilling test15 established malabsorption of B12 (0.8% of the dose excreted in 24 hours) which was corrected to 11% of the dose by coadministration of IF. Her serum lacked anti–intrinsic factor autoantibodies. Informed consent according to the Declaration of Helsinki was obtained from the patient and the mother for additional studies to identify the cause of the disorder.
Analyses of gastric juice
Gastric juice was collected following pentagastrin stimulation, pepsin was inactivated by raising the pH to 10, and, after lowering to pH 7, stored at –20°C. The Cbl-binding capacity in the gastric juice was measured by a competitive binding assay,16 and the specific binding of Cbl by IF was determined by preincubating the gastric juice with cobinamide (Cbi) to saturate the haptocorrin (HC).17 Cbl-binding proteins in the gastric juice were also analyzed by gel filtration chromatography of protein bound [57Co]Cbl and by immunoprecipitation of protein bound [57Co]Cbl by using antiserums specific for IF and for HC, generated in our laboratory. These antiserums were also used to quantify immunoreactive IF and HC in the gastric juice by using radioimmunoassay (RIA) protocols similar to that described for measuring TCII in serum.18
Analysis of genomic DNA
Genomic DNA was isolated from the peripheral blood of the patient and her mother. Exons encoding the IF protein were amplified by the polymerase chain reaction (PCR) by using primer pairs corresponding to the intronic regions flanking each exon.8 Genomic DNA (1 μg), deoxynucleotide triphosphate (dNTP) nucleotides (0.2 mM each), 2.5 U Taq polymerase, and 10 pmol forward and reverse primers in a volume of 50 μL were subjected to 30 cycles of denaturation, annealing, and extension at 94°C, 58°C, and 72°C, respectively, each for 1 minute. The PCR product was separated in a 2% agarose gel, the amplified fragment was excised, and the DNA was recovered. The nucleotide sequence of each amplified fragment was determined by the dideoxy chain termination reaction.19
Results and discussion
The onset of megaloblastic anemia at an early age with IF lacking in the gastric juice and normal acid output following pentagastrin administration (Table 2), is characteristic of hereditary IF deficiency. An abnormal Schilling test that was corrected by coadministration of IF provided additional evidence that the Cbl malabsorption was due to lack of IF. The inhibition of Cbl binding in the gastric juice by Cbi indicated absence of IF in the patient's gastric juice (Table 2), and this inhibition was confirmed by the absence of a [57Co]Cbl-labeled protein peak corresponding to IF by size exclusion chromatography (Figure 1A). The protein peak observed with the patient's gastric juice was identified as HC by its larger size and by immunoprecipitation of the [57Co]Cbl-labeled protein with an antiserum to HC (Figure 1B). There was no immunoreactive IF protein in the gastric juice by RIA using a monospecific polyclonal antiserum to human IF. With the exception of functionally abnormal IF proteins that have been identified,12-14 most patients either lack an intact protein or have a partially degraded IF protein.20
Identification of Cbl binding protein(s) in gastric juice. (A) Fractionation of protein bound [57Co]Cbl in the gastric juice by size exclusion chromatography on a Sephacryl S-200 column (Amersham Pharmacia, Piscataway, NJ). The ratio of Ve/Vo (elution volume/void volume) is plotted against protein bound radioactivity per fraction. The protein peaks marked HC and IF correspond, respectively, to the elution of haptocorrin and intrinsic factor from the column. (B) Immunoprecipitation of protein bound radioactivity with antiserum specific for IF and for HC.
Identification of Cbl binding protein(s) in gastric juice. (A) Fractionation of protein bound [57Co]Cbl in the gastric juice by size exclusion chromatography on a Sephacryl S-200 column (Amersham Pharmacia, Piscataway, NJ). The ratio of Ve/Vo (elution volume/void volume) is plotted against protein bound radioactivity per fraction. The protein peaks marked HC and IF correspond, respectively, to the elution of haptocorrin and intrinsic factor from the column. (B) Immunoprecipitation of protein bound radioactivity with antiserum specific for IF and for HC.
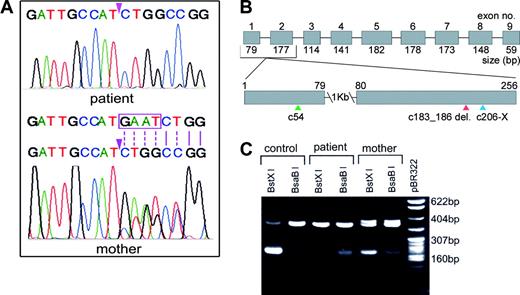
Analysis of exons amplified by PCR showed normal nucleotide sequences except for exon 2. There was a 4-base deletion spanning nucleotides 104 to 107 (GAAT) in exon 2 (Figure 2A, upper panel), generating a frame shift in the codons and premature termination of translation 20 bases downstream (Figure 2B). This sequence predicts a translated product of 67 amino acids of which the last 7 are changed and must be unstable because no immunoreactive IF was detected in the gastric juice. One normal allele and one mutated allele were evident in the mother's DNA from the overlapping nucleotide peaks in the sequence immediately 3′ of the deletion (Figure 2A, lower panel). The PCR product from the mother's genomic DNA showed heteroduplex formation, appearing as a doublet in the agarose gel (Figure 2C) which is characteristic of insertions or deletions. Digestion of this PCR product with BstXI or with BsaBI generated an approximate 200-bp fragment (Figure 2C), confirming the presence of one normal and one mutated allele. The presence of the uncut fragment and low yield of the cut fragment with these enzymes were due to inefficient cutting by both enzymes which require unique conditions and temperatures of 55°C and 60°C, respectively, for activity. Because exon 2 contained only the mutated sequence, the child could be either homozygous or hemizygous. The latter seems unlikely, because Southern blot analysis of genomic DNA digested with HindIII, HinfI, and PstI that cut 3 or more times in the gene showed banding patterns and hybridization signals similar to those observed with control DNA. Moreover, the yield of the PCR product for each of the exons was also similar to that obtained for the control, suggesting the presence of both alleles in the propositus. Consanguinity could not be established because of lack of family history and DNA from the father. However, on the basis of available data, it seems likely that the patient is homozygous for the deletion and the inheritance is autosomal recessive.
Analysis of PCR-amplified exon 2 from genomic DNA. (A) Nucleotide sequence of the region with the GAAT 4-base deletion (beginning at the arrowhead) in the patient (upper panel) who is homozygous, and the corresponding region in the mother (lower panel) who is heterozygous for this deletion. The normal sequence in one allele in the mother is shown in the upper strand, and the boxed sequence in this strand is shown as deleted in the lower strand to depict the mutated allele. Note the overlapping peaks past the deletion because of dissimilar bases (indicated by broken lines) and single peaks because of identical bases (indicated by solid lines), representing one normal allele and one mutated allele in the mother. (B) Schematic representation of the IF gene (approximately 20 kb). The boxed segments represent the 9 exons (1251 bp). The region with the deletion is enlarged to show the signal peptide cleavage site (c54, green arrowhead), the location of the deletion (c183_186delGAAT, red arrowhead), and termination of translation (c206-X, blue arrowhead). (C) Restriction enzyme digestion of the PCR-amplified fragment with BstXI and BsaBI. The control has a single site for BstXI and does not cut with BsaBI; the homozygous patient has lost the site for BstXI because of the 4-base deletion which resulted in a site for BsaBI; the mother who is heterozygous shows sites for both restriction enzymes, and the heteroduplex in the uncut DNA represents the 2 alleles.
Analysis of PCR-amplified exon 2 from genomic DNA. (A) Nucleotide sequence of the region with the GAAT 4-base deletion (beginning at the arrowhead) in the patient (upper panel) who is homozygous, and the corresponding region in the mother (lower panel) who is heterozygous for this deletion. The normal sequence in one allele in the mother is shown in the upper strand, and the boxed sequence in this strand is shown as deleted in the lower strand to depict the mutated allele. Note the overlapping peaks past the deletion because of dissimilar bases (indicated by broken lines) and single peaks because of identical bases (indicated by solid lines), representing one normal allele and one mutated allele in the mother. (B) Schematic representation of the IF gene (approximately 20 kb). The boxed segments represent the 9 exons (1251 bp). The region with the deletion is enlarged to show the signal peptide cleavage site (c54, green arrowhead), the location of the deletion (c183_186delGAAT, red arrowhead), and termination of translation (c206-X, blue arrowhead). (C) Restriction enzyme digestion of the PCR-amplified fragment with BstXI and BsaBI. The control has a single site for BstXI and does not cut with BsaBI; the homozygous patient has lost the site for BstXI because of the 4-base deletion which resulted in a site for BsaBI; the mother who is heterozygous shows sites for both restriction enzymes, and the heteroduplex in the uncut DNA represents the 2 alleles.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-07-2239.
Supported by the Raymond and Frances Church Biomedical Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Identification of Cbl binding protein(s) in gastric juice. (A) Fractionation of protein bound [57Co]Cbl in the gastric juice by size exclusion chromatography on a Sephacryl S-200 column (Amersham Pharmacia, Piscataway, NJ). The ratio of Ve/Vo (elution volume/void volume) is plotted against protein bound radioactivity per fraction. The protein peaks marked HC and IF correspond, respectively, to the elution of haptocorrin and intrinsic factor from the column. (B) Immunoprecipitation of protein bound radioactivity with antiserum specific for IF and for HC.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/4/10.1182_blood-2003-07-2239/6/m_zh80040456750001.jpeg?Expires=1770802015&Signature=3HFFJd2Hq~OTcnsLRfMjqKuEkePCtLXh36MCLItx6fHO9337Q7~qJdH8cAl92WFrFlKaCe6Q1iHUvFbvfycWpSQjIBBS7nZ-0K4NoU~ksxF0~AzjJyrmcpqH5EiehdGpQ7CcZPkLZlfnmXGDmInnTecHl~LQydaw9Uo0IoootG-KdKmu24bKEGkqJwfKjzWf04k-WHqvcJXoW2FCObFRDbC~W-JNla5mylEQpjlxPZouC3c4VbCU2awTkSfKJc907SNrxh3tucSm1-oC76G0lHOxo7adyucv4mq0LzxaU7fy0Fd1DpL9YUcS3iMyRoOyfZZeD5kA3hVoXEc2TkS2ag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)