Abstract
The major challenge in allogeneic hematopoietic cell transplantation is how to transfer allogeneic T-cell immunity without causing graft-versus-host disease (GVHD). Here we report a novel strategy to selectively prevent GVHD by depleting CD62L+ T cells (naive and a subset of memory T cells). In unprimed mice, CD62L– T cells (a subset of memory T cells) failed to proliferate in response to alloantigens (which the mice have never previously encountered) and were unable to induce GVHD in allogeneic hosts. CD62L– T cells contributed to T-cell reconstitution by peripheral expansion as well as by promoting T-cell regeneration from bone marrow stem/progenitor cells. CD62L– T cells from the animals previously primed with a tumor cell line (BCL1) were able to inhibit the tumor growth in vivo but were unable to induce GVHD in the third-party recipients. This novel technology may allow transfer of allogeneic recall antitumor and antimicrobial immunity without causing GVHD.
Introduction
Allogeneic hematopoietic cell transplantation has long been recognized as a curative treatment choice for a variety of hematopoietic malignancy diseases.1 Mature T cells contained in the graft play a critical role in the success of this treatment because depletion of mature T cells from the graft significantly increases the risk of graft failure and leukemia relapse and delays immune reconstitution.2 On the other hand, mature T cells also mediate graft-versus-host disease (GVHD),3 which is a potentially fatal side effect associated with allogeneic hematopoietic cell transplantation. Depletion of all mature T cells from the graft is very effective in preventing GVHD but at the same time all mature T cell–mediated beneficial effects are lost.2 The major challenge is how to transfer allogeneic T-cell immunity without causing GVHD.4
Alloreactive T cells, which represent only a small portion of T cells,5,6 are responsible for induction of GVHD.3 Theoretically, selective depletion of alloreactive T cells may prevent GVHD while preserving other immune functions. Indeed, GVHD has been successfully prevented without impairing other immune functions in several animal models mainly by targeting activation markers after host-reactive T cells are activated ex vivo7-11 or in vivo.12 Likewise, similar strategies were also successfully tested in vitro in humans.13-17 However, only a few of these strategies18,19 have been explored in human trials so far, probably due to the complexity of these published approaches. Development of simpler and effective approaches to transfer allogeneic T-cell immunity without causing GVHD is still highly desirable.4
Naive T cells (CD62L+) are T cells that have never encountered antigens specific for their T-cell receptor.20-22 Memory/activated T cells (CD62L– or CD62L+) are T cells that have previously been exposed to their corresponding antigens.20,21 If a donor has never encountered the host alloantigens, GVHD-inducing host-reactive T cells should be contained in naive T-cell compartments. If this hypothesis is correct as suggested by several published studies,5,23 CD62L– T cells, which are devoid of naive T cells and represent a subset of memory/activated T cells,20-22,24-27 should not be able to induce GVHD.
In the current study, we compared the ability of CD62L– T cells to induce GVHD with unseparated as well as CD62L+ T cells (represent naive and a subset of memory/activated T cells20,21 ). Moreover, the effects of CD62L– T cells on T-cell reconstitution and whether CD62L– T cells can carry functional antitumor immunity were also examined.
Material and methods
Mice
BALB/c (H2d, Mls-2a, Mls-3a), C57BL/6 (H2b, Mls-2b, Mls-3b, CD45.2, Thy1.2), C3H/HeJ (H2k, Mls-2a, Mls-3a), and B6 severe combined immunodeficiency (SCID) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The breeders of congenic C57BL/6 CD45.1 mice (H2b, Mls-2b, Mls-3b, CD45.1, Thy1.1) were kind gifts from Dr Jos Domen (Duke University, Durham, NC). Only female mice were used in this study. The unprimed donor mice were 10 to 19 weeks old. For primed animals, the mice were 7 to 11 weeks old at the time of immunization. The recipient mice and the normal control mice were 8 to 12 weeks old when given the transplants.
BCL1 cell lines
BCL1 is a spontaneous B-cell leukemia/lymphoma cell line of BALB/c origin.28 An in vitro line and an in vivo line7 (both are kind gifts from Drs Defu Zeng and Samuel Strober, Stanford University, CA) were used in this study. The in vitro line was maintained in culture and used for all in vitro studies. The in vivo line was maintained by serial passage in normal BALB/c mice. Purified BCL1 cells, which were positively selected by anti-BCL1 idiotype antibody7 (clone 6A5, a generous gift from Dr Ellen S. Vitetta, University of Texas, Dallas) using magnetic beads (Miltenyi Biotec, Auburn, CA) from spleen cells from BCL1-bearing mice, were used for in vivo tumor challenge assays.
Immunization
C57BL/6 mice were given intraperitoneal injections of 5 × 107 irradiated (100 Gy) BCL1 cells from spleens of BCL1-bearing BALB/c mice. The primed animals were used 12 to 14 days after immunization.
Cell separation
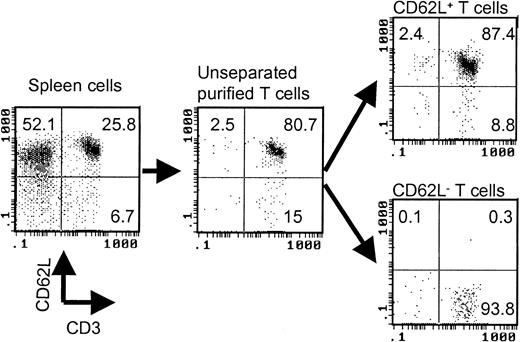
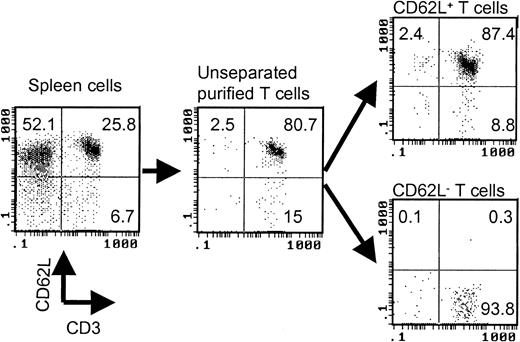
T cells were first negatively selected from spleen cells by using antimajor histocompatibility complex (MHC) class II (Ia), CD11a, and DX5 microbeads (Miltenyi Biotec). Anti-CD62L microbeads (Miltenyi Biotec) were then used to separate purified T cells into CD62L+ and CD62L– fractions. The purity of the cell population was always checked by flow cytometry after cell separation. A typical cell separation is shown in Figure 1. The responder cell doses were always adjusted based on T-cell content.
Separation of CD62L– T cells by magnetic beads. The values represent percentages of total cells in each cell fraction.
Separation of CD62L– T cells by magnetic beads. The values represent percentages of total cells in each cell fraction.
Mixed lymphocyte reaction assay
Various numbers of responder spleen cells (immune reconstitution: 2.5 × 105 spleen cells; other assays: 0.0156-2.5 × 105 purified T cells) were plated in flat-bottom 96-well culture plates with 5 × 105 irradiated (20 Gy) spleen stimulator cells or 2.5 × 105 irradiated (100 Gy) BCL1 cells in a final volume of 200 μL. After 96 hours of incubation at 37°C and 5% CO2, cultures were pulsed with 1 μCi (0.037 MBq) [3H]-thymidine per well. After another 16 hours of incubation, the cultures were harvested and counted in a MicroBeta Trilux liquid scintillation counter (EG&G Wallac, Turku, Finland). Triplicate cultures were set up for every cell population tested. The data using 1.25 × 105 responder T cells were presented because the peak responses were observed for all unseparated T cell–mediated responses and most of the CD62L+ T cell–mediated responses in this dose of responder T cells. The data were presented as mean ± SD of triplicate wells.
GVHD models
One million T cells were injected intravenously together with 1 × 107 T cell–depleted bone marrow29 (TCD BM) cells via tail vein into lethally irradiated BALB/c (8.5 Gy) or C3H/HeJ (9.5 Gy) recipients. Full donor chimerism (> 95% donor) was established in all recipients as detected by anti–H-2Db antibody29 (data not shown). Recipients were weighed once every 7 days. Survival and clinical evidence of GVHD such as skin changes (hair loss and erythema), diarrhea, and posture were monitored daily. Biopsies were taken from skin, intestine, and liver for histologic analyses for evidence of GVHD when the animals were moribund.
BCL1 tumor model
T cells from C57BL/6 mice were injected together with 1 × 104 purified BCL1 cells via tail vein into B6 SCID mice. The presence of BCL1 cells was monitored in peripheral blood using an anti-BCL1 idiotype antibody7 on days +14, +28, +35, +42, +56, and +70. Evidence of tumor growth was also documented by the presence of tumor nodules and spleen weight. The survival was monitored daily.
Flow cytometric analysis
Blood (50 μL) or 1 × 106 cells were incubated with titrated antibodies for 15 minutes at room temperature in the dark. To lyse red cells in the whole-blood samples, the stained whole blood was then processed in Multi-Q-Prep (Coulter, Miami, FL). The stained cells were analyzed using a Coulter EPICS XL-MCL flow cytometer equipped with SYSTEM II software (Coulter). The absolute counts were determined by using Flow-Count fluorospheres29 (Coulter). The antibodies included fluorescein isothiocyanate (FITC)–conjugated anti-CD62L (clone MEL-14); R-phycoerythrin (PE)–conjugated anti-CD45.1 (clone A20); cy-chrome–conjugated anti-CD44 (clone IM7), and their isotype controls from BD PharMingen (San Diego, CA); FITC-conjugated anti–H-2Db (clone CTDb); PE-conjugated anti-CD4 (clone CT-CD4), B220 (clone RA3-6B2); PE-Texas red–conjugated anti-CD4 (clone RM4-5; tri-color–conjugated anti-CD4 (clone CT-CD4), CD8α (clone CT-CD8α), and their isotype controls from Caltag (South San Francisco, CA). T-cell repertoire was determined by a kit (mouse Vβ TCR screening panel) from BD PharMingen.
Mouse sjTREC assay
Statistical analysis
Data were presented as mean ± SD. Comparison between groups was performed by either t test (2 groups) or analysis of variance (> 2 groups). Survival data were analyzed by log-rank test. All statistical analyses were done using Statview software (SAS Institution, Cary, NC). P values less than .05 were considered significant.
Results
Effects of CD62L– T cells from unprimed mice on GVHD
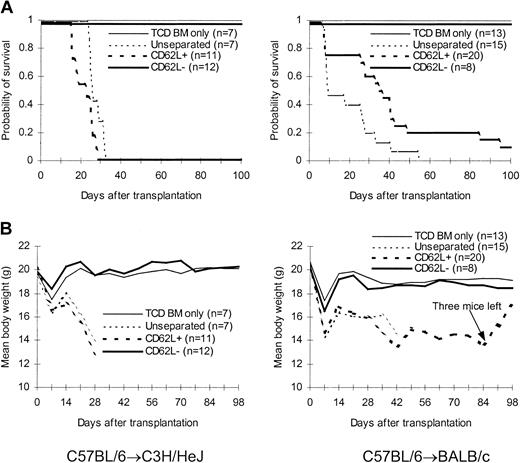
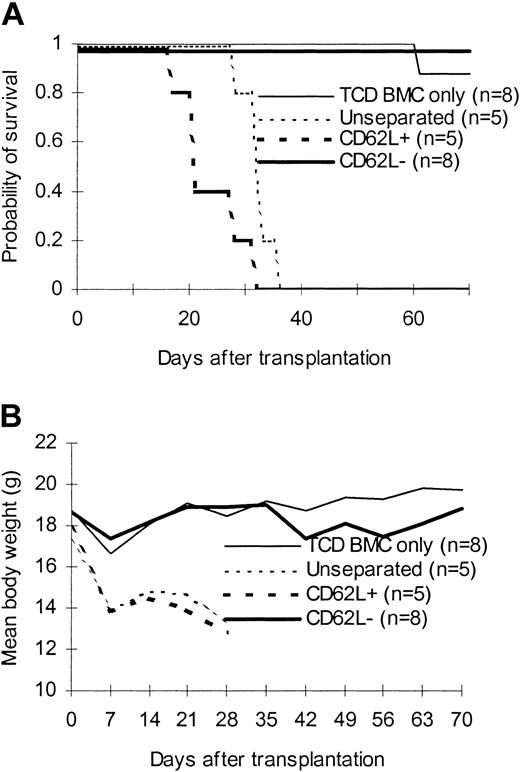
We first tested the ability of CD62L– T cells obtained from unprimed C57BL/6 mice to proliferate in response to alloantigens and tumor antigens using a proliferation assay. The ability of CD62L– T cells to proliferate was only 2.8%, 0.4%, and 0.2% of those of unseparated T cells in response to BALB/c, C3H/HeJ, and BCL1 (a BALB/c-origin lymphoma/leukemia cell line) antigens, respectively (Figure 2A). Similar results were observed when BALB/c mice were used as donors (Figure 2B). We then tested whether CD62L– cells from unprimed C57BL/6 mice, which have never seen the host antigens before, could induce GVHD in 2 different MHC-mismatched hosts (C3H/HeJ and BALB/c). One million T cells were transplanted into lethally irradiated hosts along with 1 × 107 TCD BM cells. All C3H/HeJ recipients of unseparated and CD62L+ T cells developed GVHD (Figure 3) and died by day 35 after transplantation (Figure 3A). By contrast, none of CD62L– T-cell recipients died more than 100 days after transplantation (Figure 3A). Similar to TCD BM controls, none of CD62L– T-cell recipients developed GVHD as documented by body weight (Figure 3B) and histology (Figure 4D) more than 100 days after transplantation. Similar results were obtained in BALB/c recipients (Figure 3). CD62L– T cells are at least 10 times less potent to induce GVHD than unseparated T cells because as few as 1 × 105 purified C57BL/6 T cells could induce GVHD in BALB/c recipients (B.J.C., unpublished observation, June 2000). These data clearly demonstrated that CD62L– T cells (a subset of memory/activated T cells) from unprimed mice are unable to induce GVHD in MHC-mismatched hosts.
Proliferation assay. Purified T-cell responders were cultured with irradiated allogeneic spleen cells for 112 hours. Proliferation was determined by [3H]-thymidine incorporation. (A) Unprimed C57BL/6 responder. This is a representative sample of 4 independent similar experiments. (B) Unprimed BALB/c responder. *P < .01, CD62L– versus other groups. All values represent means ± SD of triplicate wells.
Proliferation assay. Purified T-cell responders were cultured with irradiated allogeneic spleen cells for 112 hours. Proliferation was determined by [3H]-thymidine incorporation. (A) Unprimed C57BL/6 responder. This is a representative sample of 4 independent similar experiments. (B) Unprimed BALB/c responder. *P < .01, CD62L– versus other groups. All values represent means ± SD of triplicate wells.
Effect of CD62L– T cells from unprimed animals on GVHD. T cells were transplanted into lethally irradiated allogeneic host along with T cell–depleted bone marrow cells. The data were pooled from 2 to 3 independent experiments. (A) Survival. P < .0001, CD62L– versus unseparated or CD62L+. (B) Weight curve.
Effect of CD62L– T cells from unprimed animals on GVHD. T cells were transplanted into lethally irradiated allogeneic host along with T cell–depleted bone marrow cells. The data were pooled from 2 to 3 independent experiments. (A) Survival. P < .0001, CD62L– versus unseparated or CD62L+. (B) Weight curve.
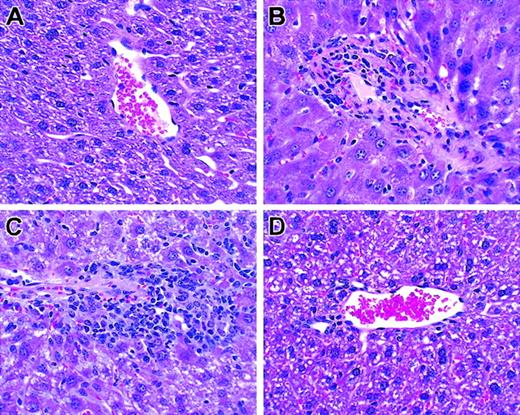
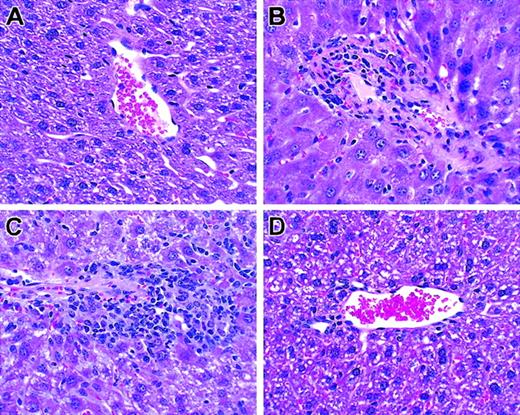
Histologic analysis of liver. T cells from unprimed C57BL/6 mice were transplanted into lethally irradiated BALB/c mice along with T cell–depleted bone marrow cells. Biopsies were obtained on day +26. Hematoxylin and eosin; original magnification × 100. (A) TCD BM only. (B) Unseparated. (C) CD62L+. (D) CD62–.
Histologic analysis of liver. T cells from unprimed C57BL/6 mice were transplanted into lethally irradiated BALB/c mice along with T cell–depleted bone marrow cells. Biopsies were obtained on day +26. Hematoxylin and eosin; original magnification × 100. (A) TCD BM only. (B) Unseparated. (C) CD62L+. (D) CD62–.
Effects of CD62L– T cells from unprimed mice on T-cell reconstitution
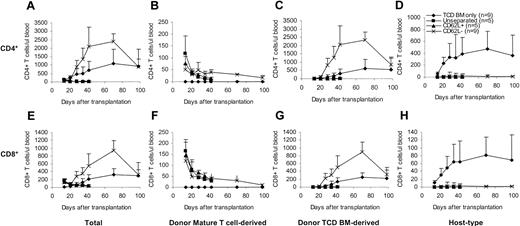
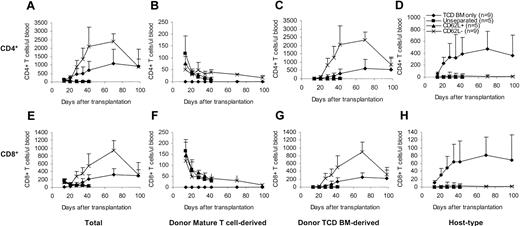
To determine whether and how CD62L– T cells contribute to the T-cell reconstitution after transplantation, we transplanted T cells from congenic C57BL/6 CD45.1 mice along with C57BL/6 TCD BM into lethally irradiated BALB/c mice and followed the T-cell recovery at various time points after transplantation. The origins of T cells can be distinguished by using anti-CD45.1 and anti–H-2Db antibodies31 (donor mature T cell–derived: CD45.1+H-2Db+; donor TCD BM-derived: CD45.1–H-2Db+; host-type: CD45.1–H-2Db–). In contrast to unseparated and CD62L+ T cells, CD62L– T cells dramatically accelerated both total CD4+ and CD8+ T-cell recovery (Figure 5A,E). Similar to unseparated and CD62L+ T cells, peripheral expansion of CD62L– T cells contributed to T-cell recovery (Figure 5B,F). Unlike unseparated and CD62L+ T cells, CD62L– T cells promoted T-cell recovery by stimulating the regeneration of T cells from donor TCD BM (Figure 5C,G). Addition of CD62L– T cells greatly lowered the numbers of residual host-type T cells (Figure 5D,H), thus dramatically increasing the degrees of donor chimerism (data not shown). By day +98, total T cells in CD62L– T-cell recipients recovered back to normal levels (Figure 5A,E).
Effects of CD62L– T cells on T-cell reconstitution after hematopoietic cell transplantation. T cells from congenic C57BL/6 CD45.1 mice were transplanted into lethally irradiated BALB/c mice along with C57BL/6 TCD BM. Reconstitution of T cells of different origins was followed by flow cytometric analysis. Only the P values from the comparison between CD62L– and TCD BM only are indicated. All values represent means ± SD. (A) Total CD4+; P < .05, except on day +14 and day +98. (B) Donor mature T cell–derived CD4+; P < .01. (C) Donor TCD BM–derived CD4+; P < .01 except on day +98. (D) Host-type CD4+; P < .01. (E) Total CD8+; P < .05 except on day +98. (F) Donor mature T cell–derived CD8+; P < .05. (G) Donor TCD BM–derived CD8+; P < .05 except on day +98. (H) Host-type CD8+; P < .01.
Effects of CD62L– T cells on T-cell reconstitution after hematopoietic cell transplantation. T cells from congenic C57BL/6 CD45.1 mice were transplanted into lethally irradiated BALB/c mice along with C57BL/6 TCD BM. Reconstitution of T cells of different origins was followed by flow cytometric analysis. Only the P values from the comparison between CD62L– and TCD BM only are indicated. All values represent means ± SD. (A) Total CD4+; P < .05, except on day +14 and day +98. (B) Donor mature T cell–derived CD4+; P < .01. (C) Donor TCD BM–derived CD4+; P < .01 except on day +98. (D) Host-type CD4+; P < .01. (E) Total CD8+; P < .05 except on day +98. (F) Donor mature T cell–derived CD8+; P < .05. (G) Donor TCD BM–derived CD8+; P < .05 except on day +98. (H) Host-type CD8+; P < .01.
Effects of CD62L– T cells from BCL1-primed mice on antitumor response and anti–third-party GVHD
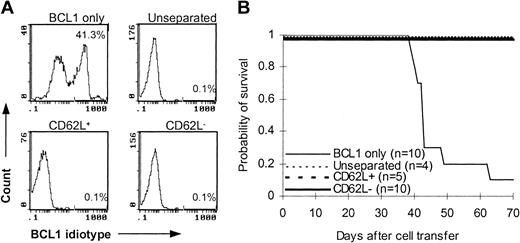
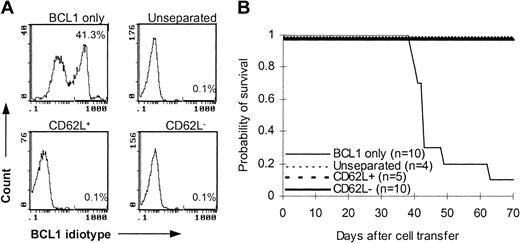
To determine whether CD62L– T cells preserve memory T-cell immunity, we used C57BL/6 mice that were immunized with BCL1 cells 12 to 14 days earlier as T-cell donors. After injection with 1 × 104 purified BCL1 cells, 9 of 10 B6 SCID mice developed leukemia as detected by flow cytometry (Figure 6A) and lymphoma as evidenced by tumor nodules in liver and spleen (data not shown) and died between day 39 and day 63 after tumor challenge (Figure 6B). Similar to unseparated and CD62L+ T cells, addition of CD62L– T cells in the inoculum completely prevented B6 SCID mice from developing tumor (Figure 6A). All CD62L– T-cell recipients survived more than 70 days after BCL1 cell injection (Figure 6B). These data suggested that CD62L– T cells from BCL1-primed mice preserved the responses against BCL1 tumor cells.
Antitumor effect of CD62L– T cells from BCL1-primed C57BL/6 mice. T cells from BCL1-primed C57BL/6 mice were injected together with 1 × 104 purified BCL1 cells into B6 SCID mice. Development of BCL1 leukemia was monitored by flow cytometry using anti-BCL1 idiotype antibody. (A) Flow cytometric analysis of BCL1 cells in peripheral blood. The percentages represent BCL1 cells in peripheral blood obtained on day 35. (B) Survival; P < 001, BCL1 only versus other groups.
Antitumor effect of CD62L– T cells from BCL1-primed C57BL/6 mice. T cells from BCL1-primed C57BL/6 mice were injected together with 1 × 104 purified BCL1 cells into B6 SCID mice. Development of BCL1 leukemia was monitored by flow cytometry using anti-BCL1 idiotype antibody. (A) Flow cytometric analysis of BCL1 cells in peripheral blood. The percentages represent BCL1 cells in peripheral blood obtained on day 35. (B) Survival; P < 001, BCL1 only versus other groups.
Effects of CD62L– T cells from BCL1-primed mice on anti–third-party GVHD
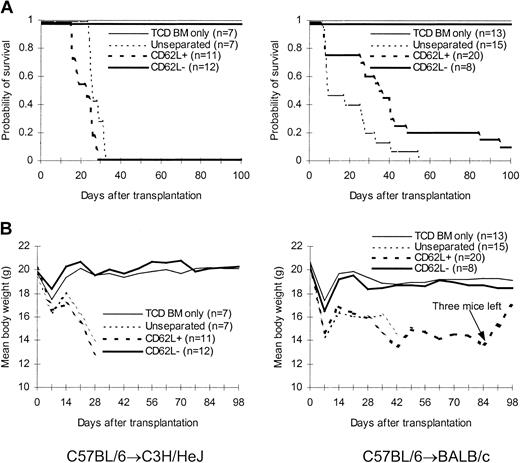
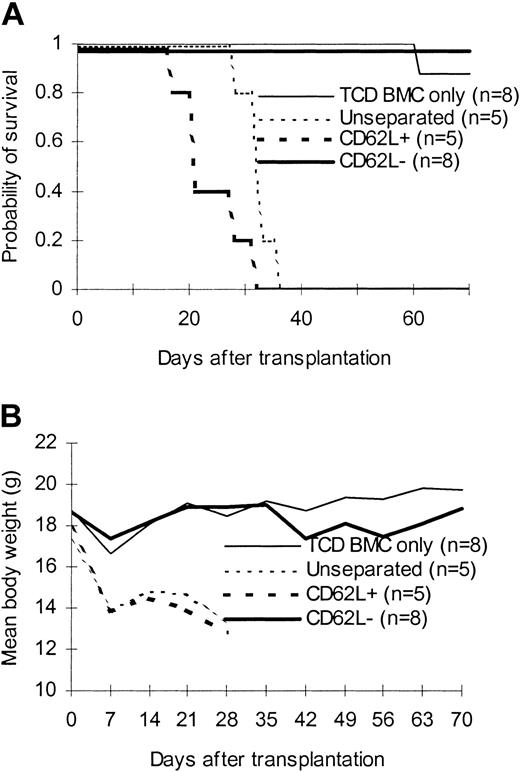
To confirm that only BCL1-specific but not third party–specific T cells lost the expression of CD62L molecules after priming with BCL1 cells, we transferred CD62L– T cells from BCL1-primed C57BL/6 mice into lethally irradiated third-party C3H/HeJ mice to determine whether they can induce GVHD. As expected, CD62L– T cells from BCL1-primed C57BL/6 mice were unable to induce GVHD in C3H/HeJ mice (Figure 7). All CD62L– T-cell recipients survived more than 70 days (Figure 7B). By contrast, all unseparated and CD62L+ T-cell recipients developed severe GVHD and died within 40 days after transplantation (Figure 7).
Effect of CD62L– T cells from BCL1-primed animals on anti–third-party GVHD. T cells from BCL1-primed C57BL/6 mice were transplanted along with T cell–depleted bone marrow into lethally irradiated third-party C3H/HeJ mice. (A) Survival; P < .001, CD62L– versus unseparated or CD62L+. (B) Weight curve; P < .001, CD62L– versus other groups.
Effect of CD62L– T cells from BCL1-primed animals on anti–third-party GVHD. T cells from BCL1-primed C57BL/6 mice were transplanted along with T cell–depleted bone marrow into lethally irradiated third-party C3H/HeJ mice. (A) Survival; P < .001, CD62L– versus unseparated or CD62L+. (B) Weight curve; P < .001, CD62L– versus other groups.
Long-term immune reconstitution of CD62L– T-cell recipients
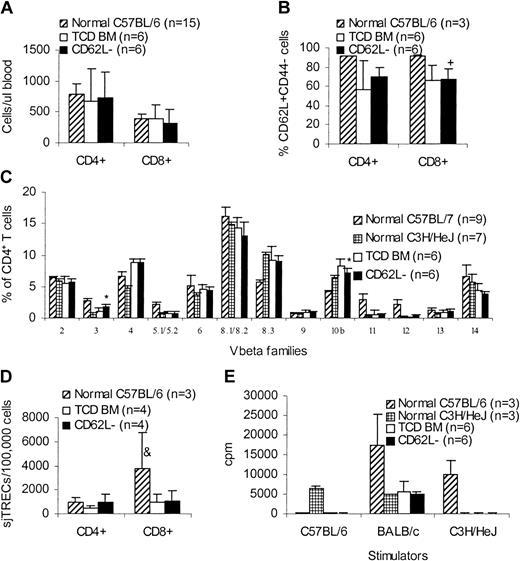
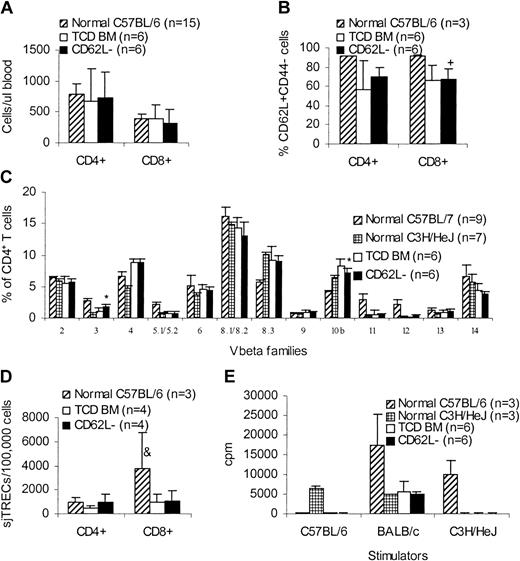
One of the concerns with this approach is the skewing of T-cell repertoire after transfer of CD62L– T cells because CD62L– T cells do not contain naive T cells. We therefore evaluated the long-term immune reconstitution in CD62L– T-cell recipients. More than 100 days after transplantation, C3H/HeJ recipients (Figure 3) of unprimed C57BL/6 CD62L– T cells were killed and peripheral blood and spleen cells were obtained for phenotypic and functional assays in comparison with TCD BM controls and normal mice. CD62L– T-cell recipients had similar amounts of CD4+ and CD8+ T cells (Figure 8A) as well as CD62L+CD44– naive T cells (Figure 8B) in peripheral blood compared to TCD BM recipients. Similar results were obtained in spleen (data not shown). The lower percentages of naive T cells in transplant recipients (Figure 8B) may be due to the older ages compared to normal donor mice. Normal peripheral T-cell counts in CD62L– T-cell recipients might have resulted from recovered thymopoiesis as demonstrated by similar sjTRECs (Figure 8D) and similar T-cell repertoire (Figure 8C) compared to TCD BM recipients. Similar to TCD BM recipients, CD62L– T-cell recipients could not respond to either donor-type (C57BL/6) or recipient-type (C3H/HeJ) antigens, but retained the ability to respond to third-party (BALB/c) antigens in mixed lymphocyte reaction (MLR; Figure 8E), indicating that antigen-specific immune tolerance was established in CD62L– T-cell recipients. Antigen-specific tolerance was at least partially a result of clonal deletion because Vβ5.1/5.2+ T cells recognizing Mls-2 and Mls-3 antigens, which are present in recipient C3H/HeJ mice but are absent in the donor C57BL/6 mice, were deleted in CD62L– T-cell recipients (Figure 8C). However, clonal anergy might also be involved because Vβ3+ T cells, which also recognize Mls-2 and Mls-3 antigens, were not deleted in CD62L– T-cell recipients (Figure 8C). Establishment of antigen-specific immune tolerance further demonstrated the lack of GVHD in CD62L– T-cell recipients.
Long-term immune reconstitution of CD62L– T-cell recipients. More than 100 days after transplantation, C3H/HeJ recipients of unprimed C57BL/6 CD62L– T cells (Figure 3) were killed and peripheral blood and spleen cells were obtained for phenotypic and functional assays in comparison with TCD BM controls and normal mice. All values represent means ± SD. (A) Peripheral blood T-cell counts. (B) Percentages of naive T cells in peripheral blood. +P < .05, compared with normal C57BL/6. (C) CD4+ T-cell repertoire. *P < .001, CD62L– versus TCD BM; for Vβ3, P ≤ .0001, CD62L– versus normal C57BL/6 or normal C3H/HeJ; for Vβ5.1/5.2, Vβ11, and Vβ12, P < .0001, CD62L– versus normal C57BL/6. Similar results were observed on CD8+ T cells. (D) sjTREC assay. &P < .05, compared with other groups. (E) MLR assay. For C57BL/6 stimulator, P < .0001, CD62L– versus normal C3H/HeJ; for BALB/c stimulator, P < .001, CD62L– versus normal C57BL/6; for C3H/HeJ stimulator, P < .0001 CD62L– versus normal C57BL/6.
Long-term immune reconstitution of CD62L– T-cell recipients. More than 100 days after transplantation, C3H/HeJ recipients of unprimed C57BL/6 CD62L– T cells (Figure 3) were killed and peripheral blood and spleen cells were obtained for phenotypic and functional assays in comparison with TCD BM controls and normal mice. All values represent means ± SD. (A) Peripheral blood T-cell counts. (B) Percentages of naive T cells in peripheral blood. +P < .05, compared with normal C57BL/6. (C) CD4+ T-cell repertoire. *P < .001, CD62L– versus TCD BM; for Vβ3, P ≤ .0001, CD62L– versus normal C57BL/6 or normal C3H/HeJ; for Vβ5.1/5.2, Vβ11, and Vβ12, P < .0001, CD62L– versus normal C57BL/6. Similar results were observed on CD8+ T cells. (D) sjTREC assay. &P < .05, compared with other groups. (E) MLR assay. For C57BL/6 stimulator, P < .0001, CD62L– versus normal C3H/HeJ; for BALB/c stimulator, P < .001, CD62L– versus normal C57BL/6; for C3H/HeJ stimulator, P < .0001 CD62L– versus normal C57BL/6.
Discussion
Our observations demonstrate that CD62L– T cells from either unprimed (Figures 3 and 8) or nonhost antigen–primed mice (Figure 7) do not induce GVHD in MHC-mismatched allogeneic hosts. CD62L– T-cell recipients survived long-term without GVHD (Figure 3), immunologically normal (Figure 8) and specifically tolerated to host antigens as a result of clonal deletion and clonal anergy (Figure 8). After priming, CD62L– T cells carry memory immune responses that we desire (Figure 6) without causing GVHD (Figure 7). Our conclusion is consistent with data from a recent publication by Anderson et al32 demonstrating that CD4+ memory (CD62L–CD44+) T cells do not induce GVHD in a minor antigen-matched model. Our findings extend the conclusion from CD4+ T cells to CD8+ T cells because as few as 1 × 105 purified CD8+ T cells were able to induce GVHD in a C57BL/6 → BALB/c model33 and we did not observe GVHD in the recipients (Figure 3) receiving as many as 1 × 106 CD62L– T cells (contain about 2 × 105 CD8+CD62L– T cells). Furthermore, our data suggest that the conclusion can be extended from MHC-matched32 to MHC-mismatched setting. This observation seems contradictory to the data obtained by studying T-cell clones specific for nominal peptides in vitro,34 which suggest that T cells specific for an MHC antigen are in the memory T-cell population. One of the possible explanations5 is that the conclusion drawn from these in vitro assays34 using T-cell clones does not apply to the primary and even secondary immune responses in vivo. Indeed, if we revisit the classic experiments performed by Medawar,35 he demonstrated that a previously sensitized recipient only mediates a second-set rejection to the skin graft of the same MHC type but not to a third-party skin graft of different MHC. Thus, it is not surprising that memory T cells do not contain T cells specific for a particular MHC if those T cells have never been previously exposed. In addition, the data from Lonial et al36 suggest that the same concept may also apply in humans.
Naive T cells, which express CD62L, are T cells that have never encountered antigens specific for their T-cell receptors. Memory/activated T cells, which can be either CD62L– or CD62L+, are T cells that have previously been exposed to their corresponding antigens.20,21 If a donor has never encountered the host alloantigens, GVHD-inducing T cells should be contained in naive T-cell compartments. Therefore, CD62L– T cells from nonhost antigen–primed animals, which are devoid of all naive T cells and CD62L+ memory T cells, should not contain host antigen–specific T cells and are unable to induce GVHD in the allogeneic host (Figure 3). This mechanism is supported by the data from Figure 2, which demonstrated that CD62L– T cells were unable to proliferate on the challenge of alloantigens in vitro. CD4+ and CD8+ T-cell subsets have different capacities to induce GVHD in some models.37 However, these differences cannot explain the lower capacity of CD62L– T cells to induce GVHD at least in the C57BL/6 → BALB/c model (Figure 3) because CD4+ T cells play a dominant role in this model38 and CD62L– T cells have more CD4+ T cells (about 80%) than both unseparated and CD62L+ T cells have (about 50%). Wherry et al39 demonstrated that CD62L+CD8+ T cells have greater proliferative potential than CD62L–CD8+ T cells. By contrast, CD62L+CD4+ T cells were demonstrated to have lower proliferative capability than CD62L–CD4+ T cells.40 As stated previously, CD62L– T cells contained more CD4+ T cells than unseparated and CD62L+ T cells did and CD4+ T cells are the major effectors at least in the C57BL/6 → BALB/c model.38 Therefore, it is unlikely that lack of GVHD was due to less proliferative potential. Lack of GVHD in CD62L– T-cell recipients could be due to increased proportions of regulatory cells41-44 such as CD4+CD25+ and CD3+NK1.1+ cells in the graft. However, an only slightly increased percentage of CD4+CD25+ cells was found in CD62L– T cells compared to unseparated and CD62L+ T cells (4%-8% versus 8%-11%). CD3+NK1.1+ cells were not detected in the CD62L– T-cell fraction (data not shown). Moreover, the ability of CD62L– T cells from BCL1-primed mice to mediate antitumor effects (Figure 6) suggests that there is little if any activity of regulatory cells. These data suggest that lack of ability of CD62L– T cells to induce GVHD is not due to altered regulatory activity. Another possible explanation for the lack of GVHD is the decreased ability of T cells to persist in vivo.45 This is not the case for CD62L– T cells because CD62L– T cells persisted more than 70 days after transplantation (Figure 5B,F). Expression of CD62L is required for T cells to efficiently recirculate between the blood and lymph nodes.46 CD62L engages ligands expressed on high endothelial venules and triggers a signaling cascade, leading to firm arrest and lymphocyte transmigration into the lymph node.47 Therefore, lack of GVHD associated with CD62L– T cells is also possible due to the inability of CD62L– T cells to home to secondary lymphoid organs.46 However, this mechanism cannot explain the low proliferative activity of CD62L– T cells from unprimed mice in response to alloantigens in vitro. Taken together, the lower ability of CD62L– T cells to induce GVHD is most likely a result of lower frequencies of host antigen–specific T cells found in CD62L– T cells. These results seem to conflict with the results from umbilical cord blood transplantation,48-50 in which reduced incidence of GVHD was observed despite the fact that cord blood T cells contain many more naive T cells compared with adult blood.51,52 However, the lower rate of GVHD observed in recipients of umbilical cord blood may be related to functional immaturity of cord blood T cells rather than the absence of host antigen-specific T cells because even purified cord blood naive T cells do not function as well as their adult counterparts.53
It was assumed that mature T cells contribute to T-cell reconstitution mainly through peripheral expansion.54 CD62L– T cells did contribute to T-cell recovery by expansion (Figure 5B,F). Although the numbers of these early expanded cells are limited, they may be very important in mediating antitumor response (Figure 6) because they are the sole donor-type T cells in the first 3 weeks after transplantation (Figure 5). Our data (Figure 5C,G) indicate that another major contribution of CD62L– T cells is to promote the regeneration of T cells from bone marrow stem or progenitor cells most likely through thymus. This is a very surprising but important finding because in contrast to peripheral expansion,55 the repertoire of T cells generated by this pathway maintains repertoire diversity and immunocompetence to a variety of antigens.54,56 One of the possible explanations is that CD62L– T cells promote new T-cell regeneration from TCD BM by enhancing hematopoietic engraftment because even nonhost reactive cytotoxic T lymphocytes are able to facilitate engraftment of early progenitors.57
As described by many investigators,26,39,40,58,59 CD62L– T cells belong to a subset of memory T cells called “effector memory T cells.” This subset of T cells is able to mediate functional memory immune responses. Our data from Figure 6 clearly demonstrate that CD62L– T cells from BCL1-primed mice were able to completely inhibit the growth of BCL1 leukemia/lymphoma cells in vivo. This observation is in accordance with the observation by Kagamu et al that CD62L– T cells from immunized animals carry highly potent antitumor activity.60,61 In the meantime, this same population of T cells was unable to induce GVHD in the third-party recipients (Figure 7), confirming our hypothesis that memory T cells do not contain alloantigen-specific T cells if they have never encountered it before. These data suggest that it is possible to selectively prime CD62L– T cells in normal donors to carry the memory T cells that we desire without causing GVHD. In humans, it may be difficult to prime the donor using tumor cells from the recipient. However, one can use tumor-specific antigens to prime normal donor as described by Kwak et al.62 Another alternative approach is to prime donor T cells ex vivo because naive markers such as CD62L are quickly down-regulated on T cells after they encounter the antigen and get activated in vitro.63
In conclusion, our observations clearly demonstrate that allogeneic CD62L– memory/activated T cells, which carry recall T-cell immunity, do not induce GVHD and in the meantime stimulate new T-cell regeneration from bone marrow. This novel technique may allow adoptive transfer of allogeneic memory antitumor and antimicrobial immunity without causing GVHD in the context of allogeneic hematopoietic cell transplantation.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-08-2987.
Supported by National Institutes of Health grant P01-HL67314.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Defu Zeng and Samuel Strober for BCL1 cells; Dr Ellen S. Vitetta for 6A5 hybridoma; Dr Jos Domen for congenic C57BL/6 CD45.1 mice; Dr H. Kim Lyerley, Dr Timothy M. Clay, and Ms Kirsten Colling for help with ELISPOT assay; and Drs Barton F. Haynes and Gwynn D. Long for critical review of this manuscript.

![Figure 2. Proliferation assay. Purified T-cell responders were cultured with irradiated allogeneic spleen cells for 112 hours. Proliferation was determined by [3H]-thymidine incorporation. (A) Unprimed C57BL/6 responder. This is a representative sample of 4 independent similar experiments. (B) Unprimed BALB/c responder. *P < .01, CD62L– versus other groups. All values represent means ± SD of triplicate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/4/10.1182_blood-2003-08-2987/6/m_zh80040456880002.jpeg?Expires=1770143924&Signature=VWzL1Mti0vcurZV9LMfb1gKjQHbnl85R4dBa7ILLP0WiP6W77~h2HUEo7vCKzZ3dOfxYKJ0OjYt0186-K2dYaHG9-WVoJkN37YMxoSC4lR4~BEJlwzh3-VKKRcCtl79Vq6quxxnDTIUuzWCqnYk9GMYF46MvsRFsTizAkjpHvfDvjBWIFwJWFc3xwZnczv7hnx~F4AX9ciRmNmEwrrns8MPBh2fnbRwrvRjxTEUq2AAIs1d1ZN9k92QcOqYYWp65Pr3RjG8v-WVglvvo1svrRa6Rttp2WNV8WTRLk5j1DlPHB4xxINlrZvKDdB4pxhOC8532SPSZJ~J1VTJMNZ4ZqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Proliferation assay. Purified T-cell responders were cultured with irradiated allogeneic spleen cells for 112 hours. Proliferation was determined by [3H]-thymidine incorporation. (A) Unprimed C57BL/6 responder. This is a representative sample of 4 independent similar experiments. (B) Unprimed BALB/c responder. *P < .01, CD62L– versus other groups. All values represent means ± SD of triplicate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/4/10.1182_blood-2003-08-2987/6/m_zh80040456880002.jpeg?Expires=1770143925&Signature=cKpAQT-eF5~bOMIIZmhJnE1OgWjVk0XRsVh2ViIywiTl75nh~pmIgYmswjs7c-vAqUtJHJwzZwQbl6qdPRRwhqQ~xK7xoEwz3aCblHB8qVYDTrkzHQnA6YV~736v9ooPeaz-g8oPHUdYc3Itoy~wtKq8PpNXy6IE5RmkK6iH5H5vvr4fYJLzE9pdt0ikxDuwaJe80InNSok5SEl0d7qZ6Hc~TD~NoohA2Ynbwpr9jEOuXWs5G-YR8O1rwp3S2GFWwDQqC6Pf6f97zogga00fuaL9Fz5chd9QfjPArvMqoZjRqpsBWKcLEzzJD5rjgn0itQ~dm4hj5YFkKovraSM1iQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)