Abstract
Among the various chemokines that are functionally active on neutrophils, platelet factor 4 (PF-4; CXCL4) appears to have a specialized role. Lacking typical chemokine activities, PF-4 stimulates neutrophils to undergo firm adhesion to endothelial cells and, in the presence of an appropriate costimulus like tumor necrosis factor (TNF), PF-4 induces exocytosis of secondary granule contents. Analyzing the individual contribution of PF-4 and its costimuli in the control of these functions at the signaling level, we demonstrate that TNF-induced activation of p38 mitogen-activated protein (MAP) kinase (but not extracellular regulated kinase [Erk] kinases) acts as general and essential costimulatory signal in PF-4–dependent neutrophil exocytosis. This was shown by the use of a specific inhibitor (SB203580), by biologic (lipopolysaccharide, N-formyl-methionyl-leucyl-phenylalanine) and pharmacologic (anisomycin) activators of p38 MAP kinase, and by phosphorylation studies. Furthermore, TNF-mediated activation of phosphatidylinositol 3-kinase (PI 3-kinase) represents an additional essential signaling component in this process as demonstrated by studies with its inhibitor wortmannin as well as by analysis of the phosphorylation of AKT kinase. PF-4, however, directly activates src-kinases and PF-4–induced adherence as well as PF-4/TNF-mediated exocytosis was inhibited by an src-kinase inhibitor PP1. Taken together, neutrophil exocytosis and adherence are regulated on p38 MAP kinase, PI 3-kinase, and src-kinase activation.
Introduction
Platelet factor 4 (PF-4; CXCL4), a CXC chemokine synthesized by megakaryocytes and stored in platelet α granules as a noncovalent tetramer, becomes released in high concentrations on platelet activation.1,2 PF-4 shares about 30% to 60% amino acid identity with other members of the CXC chemokine family, such as neutrophil-activating peptide 2 (NAP-2; CXCL7), interleukin 8 (IL-8; CXCL8), or interferon γ-inducible protein 10 (IP-10; CXCL10). According to recent literature, CXC chemokines belong to 2 structurally different groups, depending on the presence or absence of a conserved tripeptide motif (Glu-Leu-Arg; ELR) within their N-terminus. The ELR motif has been demonstrated to be essential for the interaction of CXC chemokines with receptors CXCR1 and CXCR2 on neutrophils,3 resulting in a rapid induction of cell functions such as chemotaxis, calcium mobilization, and degranulation (for reviews, see Rollins4 and Baggiolini et al5 ). However, CXC chemokines lacking the ELR motif fail to bind to CXCR1 and CXCR2 but instead interact with other 7-transmembrane–spanning G protein–coupled receptors expressed on different cell types (for reviews, see Baggiolini et al,5 Zlotnik et al,6 and Murdoch and Finn7 ).
With regard to its functional roles as well as to its receptor usage PF-4 appears to be an exception to the family of CXC chemokines. Unlike other members of this group, which are active on a rather limited set of cell populations, PF-4 has been reported to affect a wide range of different cell types including basophils, eosinophils, endothelial cells, and fibroblasts.8-10 Moreover, we recently could show that PF-4 is also active on human monocytes, preventing these cells from undergoing spontaneous apoptosis and inducing their differentiation into a certain subtype of macrophages.11
However, PF-4 appears to be involved not only in the regulation of long-term biologic responses but can also mediate fast activation of neutrophils, a cell type that stands in the very first line of host defense. In a recent report we have shown that with regard to neutrophils highly purified PF-4 lacks several biologic activities that are typical for chemokines, such as chemotaxis, calcium mobilization, or degranulation, on these cells.12 Instead, a more specialized role for the chemokine was indicated by its requirement for a costimulus. In the presence of tumor necrosis factor (TNF), PF-4 stimulates neutrophils to undergo exocytosis of secondary granule contents or tight adhesion to different surfaces.12 Interestingly, PF-4 stimulates neither release of primary granule contents nor tertiary granules or secretory vesicles.12,13 So far, the only known function on neutrophils induced by PF-4 alone is their firm adhesion to endothelial cells.13 Investigating PF-4–binding sites on neutrophils we could demonstrate that PF-4–induced functions were not elicited by binding to IL-8 receptors or another 7-transmembrane domain molecule, but by interaction with an integral chondroitin sulfate proteoglycan.14,15
Neutrophil exocytosis of secondary granule contents appears to be a tightly controlled cellular process. However, signal transduction pathways participating in the regulation of this biologic function remain largely unclear and no data exist on signaling processes involved in PF-4–mediated activation of neutrophils. The fact that neither PF-4 nor potent stimuli like TNF alone are able to induce a relevant exocytosis response suggests the participation of more than one signaling pathway in the control of this process.
In this study we focus on the identification of intracellular signals involved in neutrophil exocytosis induced by PF-4 and TNF. We show by several lines of evidence that this function is regulated by at least 3 different signaling elements, p38 mitogen-activated protein (MAP) kinase, phosphatidylinositol 3-kinase (PI 3-kinase), and Lyn src-kinase.
Materials and methods
Materials
Human natural PF-4 was purified in our laboratory from supernatants of thrombin-stimulated platelets in a 3-step procedure, as previously described.12,14 The final PF-4 preparation exceeded 99% purity, containing no detectable protein contaminants. TNF was purchased from PeproTech (London, United Kingdom). Lipopolysaccharide (LPS), prepared from Salmonella friedenau, was a gift from Dr H. Brade (Department of Immunochemistry and Biochemical Microbiology, Research Center Borstel, Germany). N-formyl-methionyl-leucyl-phenylalanine (fMLP) and enolase were purchased from Sigma (Taufkirchen, Germany); anisomycin was from Calbiochem (Schwalbach, Germany).
Polyclonal rabbit antisera raised against p38, phospho-p38 (Thr180/Tyr182), AKT kinase, phospho-AKT kinase (Ser473), and monoclonal antiphospho-Erk (Thr202/Tyr204) antibody (clone E10) were purchased from New England BioLabs (Schwalbach, Germany). Polyclonal antisera directed against Erk1 (K-23; cross-reactive to Erk2), Lyn (44), Hck (N-30), and Fgr (N-47) were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). Horseradish peroxidase (HRP)–conjugated goat antimouse IgG and HRP-conjugated goat antirabbit IgG were from Dianova (Hamburg, Germany). Wortmannin (PI 3-kinase inhibitor, inhibitory concentration of 50% [IC50] 5 nM), SB203580 (p38 inhibitor, IC50 600 nM), PD098059 (MEK inhibitor, IC50 2 μM), PP1 (src-kinase inhibitor, IC50 Lyn 6 nM, Hck 20 nM), and SU6656 (src-kinase inhibitor, IC50 Lyn 130 nM) were purchased from Calbiochem. Protein A-agarose, Complete, Benzonase, and Pefabloc SC were obtained from Roche (Mannheim, Germany), and okadaic acid from Alexis (Grünberg, Germany).
Preparation and stimulation of neutrophils
Polymorphonuclear neutrophils were routinely isolated from citrated blood of healthy single donors by dextran sedimentation (Plasmasteril; Fresenius, Oberursel, Germany) followed by Ficoll-Hypaque (Amersham Pharmacia Biotech; Freiburg, Germany) density centrifugation as described previously.16 Neutrophils were resuspended in Dulbecco phosphate-buffered saline (D-PBS) and stored on ice until use. More than 98% of the cells were viable as assessed by trypan blue exclusion and the percentage of neutrophils exceeded 95% in all experiments as assessed by hematoxylin staining.
Cells (5 × 107/mL) were preincubated for 15 minutes at 37°C in D-PBS in the presence or absence of various inhibitors as indicated and supplemented with CaCl2 and MgCl2 to a final concentration of 0.9 mM and 0.5 mM, respectively. Subsequently, the cells were exposed for up to 30 minutes at 37°C to the different stimuli indicated. Addition of up to 0.1% dimethyl sulfoxide (DMSO; the maximum solvent concentration resulting after addition of the inhibitors) was without effect on neutrophil exocytosis, adherence, or kinase activation. Stimulations were terminated by a rapid centrifugation and supernatants were recovered for lactoferrin release while cell pellets were lysed (see “Cell lysis and Western blot analysis” or “Immunoprecipitation and in vitro phosphorylation assay”). Approval for these studies was obtained from the Institutional Review Board at the University of Lübeck (Lübeck, Germany), and informed consent was provided according to the Declaration of Helsinki.
Preparation and culture of human endothelial cells
Human endothelial cells were isolated from umbilical cord veins by collagenase treatment and cultured in dishes coated with fibronectin, as described previously.17,18 The cells were maintained in M199 (Biochrom, Berlin, Germany) supplemented with 1% penicillin/streptomycin, 1% l-glutamine (both from Biochrom), 5% fetal calf serum (FCS), 30 μg/mL endothelial cell growth factor (both from Roche), and 20 μg/mL heparin (Sigma). Cells were subcultured after trypsinization (0.5% trypsin solution, supplemented with 0.2% EDTA [ethylenediaminetetraacetic acid]; Biochrom) and used throughout passages 2 to 4.
Assays for neutrophil activation
Released lactoferrin in cell supernatants was determined by the use of a sandwich enzyme-linked immunosorbent assay (ELISA) system as described elsewhere.19 Assay backgrounds were determined in samples of unstimulated cells run in parallel and were subtracted. Determination of neutrophil adhesion to endothelial cells was performed as described in detail elsewhere.13 Briefly, neutrophils were preincubated for 20 minutes at 37°C in the presence or absence of various inhibitors as indicated and subsequently allowed to adhere to endothelial cells for 20 minutes at 37°C in the presence of 2 μM PF-4. After nonadherent cells were removed from endothelial cell layers by centrifugation, the amount of remaining cells was determined by measurement of neutrophil-specific endogenous β-glucuronidase enzymatic activity.20 Cell numbers were calculated by means of a standard of lysed cells run in parallel.
Cell lysis and Western blot analysis
Neutrophils (1 × 107 cells) were centrifuged and subsequently resuspended in 100 μL lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4, 150 mM NaCl, 1 mM EGTA [ethylene glycol tetraacetic acid], 1% NP-40, 0.25% sodium deoxycholate, 2 mM Na3VO4, 1 mM NaF, 500 nM okadaic acid, 1 × Complete, 4 mM Pefabloc SC, 250 U Benzonase, 2 mM MgCl2). After extraction for 15 minutes at 4°C, lysates were then cleared by centrifugation at 20 000g for 10 minutes at 4°C and protein concentrations were determined by the method of Bradford,21 using bovine serum albumin (BSA) as a standard (Bio-Rad, München, Germany). For Western blotting 50 μg protein was diluted in 3-fold concentrated sample buffer, and the samples were boiled for 5 minutes prior to electrophoresis. Proteins derived from cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)22 using a 10% polyacrylamide gel and blotted onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Immunodetection was performed as described in detail elsewhere.23 Bands were visualized by an enhanced chemiluminescence method (Roche) according to the manufacturer's recommendations. For reprobing, the membranes were stripped in 62.5 mM Tris-base pH 6.7, 100 mM 2-mercaptoethanol, and 2% SDS, for 30 minutes at 50°C, followed by immunodetection with the appropriate antibodies.
Immunoprecipitation and in vitro phosphorylation assay
Activation of src-kinases was determined by an in vitro phosphorylation assay using acid-denaturated enolase as exogenous substrate. Cells were stimulated as described (see “Preparation and stimulation of neutrophils”) and lysed in SrcLP (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1 mM NaF, 2 mM Na3VO4, 500 nM okadaic acid, 1 × Complete, 4 mM Pefabloc SC, 250 U Benzonase). After extraction for 15 minutes at 4°C, lysates were cleared by centrifugation (10 000g for 10 minutes at 4°C) and protein concentrations were determined by the method of Bradford,21 using BSA as a standard (Bio-Rad). Samples of cell lysate containing 300 μg (for Lyn or Hck) or 600 μg total protein (for Fgr) were incubated with 1 μg anti-Lyn, anti-Hck, or anti-Fgr antibodies, respectively, followed by precipitation with protein A-agarose. After repeated washing, beads were resuspended in kinase buffer (30 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.5, 10 mM MgCl2, 5 mM MnCl2, 1 mM CaCl2) containing 3.5 μg acid-denaturated enolase, and the kinase reaction was started by the addition of 10 μM adenosine triphosphate (ATP; in kinase buffer) and 10 μCi (0.37 MBq) γ-[32P]ATP (6000 Ci/mmol [22.0 TBq/mmol]; Hartmann Analytic, Braunschweig, Germany). The reaction was stopped after 15 minutes at 37°C by the addition of 4-fold concentrated sample buffer and boiled for 10 minutes, and proteins were separated by SDS-PAGE.22 Radioactively labeled phosphoproteins were visualized by using a PhosphorImager system (Molecular Dynamics, Krefeld, Germany).
Statistical analysis
Data are presented as mean ± SD for the number of experiments indicated in the figure legends. Statistically significant (P < .05) differences among the treatment groups were calculated using one-way analysis of variance (ANOVA) test, followed by Tukey test (for multivariant analysis), or Student paired t test (for 2-sample experiments).
Results
Effect of MAP kinase inhibitors on PF-4/TNF-induced lactoferrin release in human neutrophils
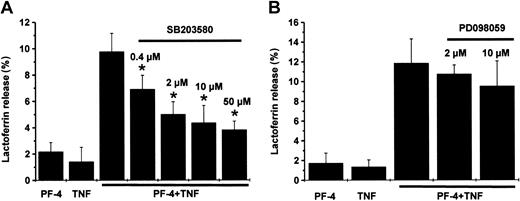
In previous studies, Mócsai et al24 could show that activation of p38 MAP kinase is involved in an fMLP-induced degranulation response in neutrophils pretreated with cytochalasin B. Therefore, in a first set of experiments we investigated the potential role of MAP kinases in the induction of secondary granule exocytosis in PF-4– and TNF-costimulated cells. To examine the contribution of p38 and Erk MAP kinases, neutrophils were preincubated in the presence or absence of increasing concentrations of inhibitors specific for p38 MAP kinase (SB203580) or MEK (PD098059), a kinase responsible for Erk MAP kinase activation. After stimulation of cells with 4 μM PF-4 and 9 ng/mL TNF, either separately or in combination, neither PF-4 nor TNF alone induced a relevant exocytosis response (up to 2.2%), as determined by the content of secondary granule marker lactoferrin in the supernatants. However, a combination of both cytokines resulted in a synergistically increased level of lactoferrin release (Figure 1A-B; 9.8% ± 1.6% and 11.8% ± 2.4%, respectively). Pretreatment of cells with SB203580 led to a dose-dependent and significant decrease of PF-4/TNF-induced exocytosis by 30% already at 0.4 μM inhibitor and 61% at 50 μM SB203580 (Figure 1A). In contrast, PD098059 had only a moderate and statistically not significant effect on this neutrophil function even at the highest concentration used (19% inhibition at 10 μM inhibitor; Figure 1B). These data provide the evidence that activation of p38 MAP kinase is involved in secondary granule exocytosis induced by PF-4 and TNF, whereas the role of Erk MAP kinases appears less dominant in this context.
Effect of MAP kinase inhibitors on PF-4/TNF-induced lactoferrin release in human neutrophils. Neutrophils were preincubated for 15 minutes in the absence or presence of increasing concentrations of (A) SB203580 (p38 inhibitor), or (B) PD098059 (MEK inhibitor), followed by stimulation with a combination of PF-4 (4 μM) and TNF (9 ng/mL) for 30 minutes at 37°C. Cells that received stimulus alone and were left without inhibitor served as controls. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds were determined in samples of unstimulated cells run in parallel were subtracted (A, 1.4% ± 1.1%; B, 1.8% ± 0.6%). Data represent mean ± SD of 5 (A) or 3 (B) independent experiments, each performed in duplicate. Asterisk (*) indicates significant differences (P < .006) between inhibitor-treated and untreated samples based on the data from 5 or 3 individual experiments.
Effect of MAP kinase inhibitors on PF-4/TNF-induced lactoferrin release in human neutrophils. Neutrophils were preincubated for 15 minutes in the absence or presence of increasing concentrations of (A) SB203580 (p38 inhibitor), or (B) PD098059 (MEK inhibitor), followed by stimulation with a combination of PF-4 (4 μM) and TNF (9 ng/mL) for 30 minutes at 37°C. Cells that received stimulus alone and were left without inhibitor served as controls. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds were determined in samples of unstimulated cells run in parallel were subtracted (A, 1.4% ± 1.1%; B, 1.8% ± 0.6%). Data represent mean ± SD of 5 (A) or 3 (B) independent experiments, each performed in duplicate. Asterisk (*) indicates significant differences (P < .006) between inhibitor-treated and untreated samples based on the data from 5 or 3 individual experiments.
TNF but not PF-4 induces phosphorylation of p38 and Erk MAP kinases in human neutrophils
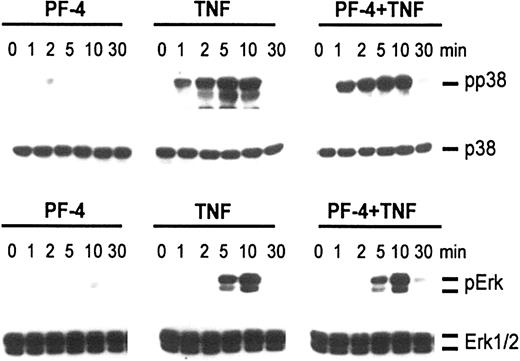
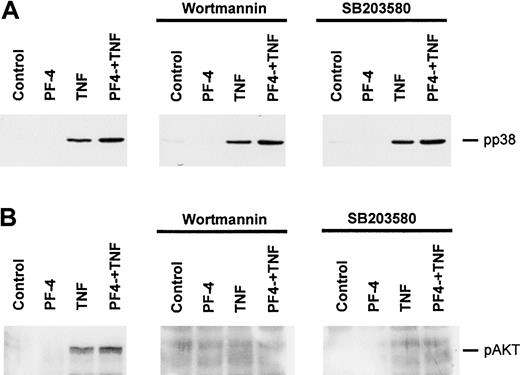
To better understand the role of MAP kinases in neutrophil exocytosis and to clarify whether PF-4 or TNF was responsible for the activation of these kinases, phosphorylation studies were performed. Cells were exposed for up to 30 minutes either to PF-4 and TNF alone or in combination. Activated cellular MAP kinases were detected by probing cell lysates with antisera against the dual-phosphorylated (activated) MAP kinases by Western blot analysis. As shown in Figure 2, stimulation with PF-4 caused phosphorylation of neither p38 nor Erk MAP kinases. However, both kinases became significantly phosphorylated in response to TNF. Whereas p38 MAP kinase was phosphorylated within 1 minute of stimulation, reaching a maximum after 5 minutes, phosphorylated Erk MAP kinases appeared first after 5 minutes and were maximal after 10 minutes of TNF treatment. Costimulation of cells with a combination of PF-4 and TNF did not change the phosphorylation patterns induced by TNF alone. It should be mentioned that one to 2 smaller bands (in addition to p38) could frequently be seen on films that were overexposed. Currently, we do not know whether these bands represent degraded or monophosphorylated forms of p38 or are due to unspecific binding of the antibodies. Such irregular bands have been observed by other authors, too, using antiphospho-p38 antisera from New England BioLabs.25,26
Phosphorylation of MAP kinases in response to stimulation with PF-4 and TNF. Neutrophils were stimulated with either PF-4 (4 μM) or TNF (9 ng/mL) alone or a combination of both stimuli for the times indicated. Activation of p38 and Erk MAP kinases was evaluated by Western blot analysis using antisera against the dual-phosphorylated (activated) MAP kinases. Blots were stripped and reprobed with p38 and Erk1/2 antisera to confirm equal protein loading. The data from one representative experiment of 5 are given.
Phosphorylation of MAP kinases in response to stimulation with PF-4 and TNF. Neutrophils were stimulated with either PF-4 (4 μM) or TNF (9 ng/mL) alone or a combination of both stimuli for the times indicated. Activation of p38 and Erk MAP kinases was evaluated by Western blot analysis using antisera against the dual-phosphorylated (activated) MAP kinases. Blots were stripped and reprobed with p38 and Erk1/2 antisera to confirm equal protein loading. The data from one representative experiment of 5 are given.
In the next set of experiments we tried to obtain further information about the potential role of Erk kinases in neutrophil exocytosis by using the MEK inhibitor PD098059. Pretreatment of cells with the inhibitor at concentration of 10 μM reduced TNF and TNF/PF-4–induced phosphorylation of Erk MAP kinases to background levels (data not shown). Taken together, these data demonstrate that TNF but not PF-4 activates p38 and Erk MAP kinases. However, the fact that PD098059 at 10 μM was able to completely abrogate phosphorylation of Erk MAP kinases but had only a minor effect on PF-4/TNF-induced exocytosis (Figure 1B) argues against a prominent role of this pathway in the induction of this function.
Activation of p38 MAP kinase is essential but not sufficient for secondary granule exocytosis in human neutrophils
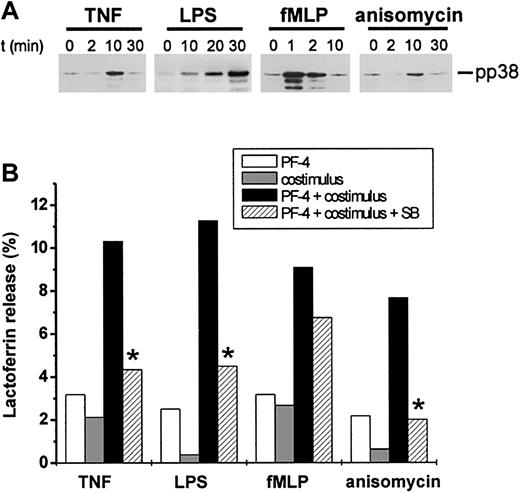
Results derived from the experiments shown above indicate that TNF-mediated activation of p38 MAP kinase plays a crucial role in the exocytosis response induced by TNF/PF-4. To test the hypothesis that this signaling pathway could represent a general costimulatory signal required for PF-4–induced exocytosis in neutrophils, we investigated different stimuli known to activate p38 MAP kinase for their potential cooperation with PF-4. Thus, neutrophils were stimulated with LPS, fMLP, anisomycin, or TNF as a positive control alone or in combination with 4 μM PF-4, and lactoferrin release rates were determined. In parallel, the capacity of each costimulus to induce a time-dependent phosphorylation of p38 MAP kinase was monitored by Western blot analysis. All 4 costimuli were able to activate p38 MAP kinase, but differed by their individual time kinetics (Figure 3A). Whereas fMLP induced a rapid response being maximal already after 1 minute, TNF- and anisomycin-mediated responses peaked after 10 minutes. LPS, however, appeared to be a rather slow activator of this signaling pathway, with phosphorylation of the enzyme becoming detectable after 10 minutes and reaching maximum after 30 minutes. Neither PF-4 nor any of the costimuli alone were able to induce a relevant exocytosis response exceeding 3.2% (Figure 3B). However, regardless of their individual time kinetics of p38 MAP kinase activation, all costimuli cooperated synergistically with PF-4 in the induction of this function as seen by release rates of 10.3% ± 0.9% (TNF), 11.3% ± 1.4% (LPS), 9.1% ± 1.4% (fMLP), or 7.7% ± 1.3% (anisomycin), respectively. Furthermore, this effect was susceptible to pretreatment of cells with 10 μM SB203580, which reduced release rates by 25% (PF-4/fMLP) and by more than 60% (PF-4/TNF, PF-4/LPS, and PF-4/anisomycin; Figure 3B).
Activators of p38 MAP kinase can serve as costimuli in PF-4–induced exocytosis. (A) Activation of p38 MAP kinase in response to different stimuli. Neutrophils were stimulated with 9 ng/mL TNF, 100 ng/mL LPS, 10 nM fMLP, or 1 μg/mL anisomycin for the times indicated and phosphorylation of p38 MAP kinase was evaluated by Western blot analysis as described in the legend to Figure 2. The data from one representative experiment of 4 are given. (B) Effect of p38 MAP kinase activators on neutrophil exocytosis. Neutrophils were preincubated for 15 minutes in the presence or absence of 10 μM SB203580 followed by stimulation with PF-4 (4 μM) or costimuli alone (9 ng/mL TNF, 100 ng/mL LPS, 10 nM fMLP, or 1 μg/mL anisomycin, respectively), or PF-4 in combination with either costimulus for 30 minutes at 37°C. Cells that received stimulus alone and were left without inhibitor served as controls. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds (1.7% ± 0.5%) determined in samples of unstimulated cells run in parallel were subtracted. Data represent the mean from 3 independent experiments (each performed in duplicate) with a variation of SD between 0.4% and 1.9%. Asterisk (*) indicates significant differences (P < .03) between inhibitor-treated and untreated samples based on the data from 3 individual experiments.
Activators of p38 MAP kinase can serve as costimuli in PF-4–induced exocytosis. (A) Activation of p38 MAP kinase in response to different stimuli. Neutrophils were stimulated with 9 ng/mL TNF, 100 ng/mL LPS, 10 nM fMLP, or 1 μg/mL anisomycin for the times indicated and phosphorylation of p38 MAP kinase was evaluated by Western blot analysis as described in the legend to Figure 2. The data from one representative experiment of 4 are given. (B) Effect of p38 MAP kinase activators on neutrophil exocytosis. Neutrophils were preincubated for 15 minutes in the presence or absence of 10 μM SB203580 followed by stimulation with PF-4 (4 μM) or costimuli alone (9 ng/mL TNF, 100 ng/mL LPS, 10 nM fMLP, or 1 μg/mL anisomycin, respectively), or PF-4 in combination with either costimulus for 30 minutes at 37°C. Cells that received stimulus alone and were left without inhibitor served as controls. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds (1.7% ± 0.5%) determined in samples of unstimulated cells run in parallel were subtracted. Data represent the mean from 3 independent experiments (each performed in duplicate) with a variation of SD between 0.4% and 1.9%. Asterisk (*) indicates significant differences (P < .03) between inhibitor-treated and untreated samples based on the data from 3 individual experiments.
From these data we conclude that activation of p38 MAP kinase represents a general and essential costimulatory signal in secondary granule exocytosis induced by PF-4 in human neutrophils. However, because p38 MAP kinase agonists alone are not able to induce a relevant exocytosis response, an additional signal provided by the PF-4 stimulus itself must exist.
PI 3-kinase is involved in PF-4/TNF-induced lactoferrin release
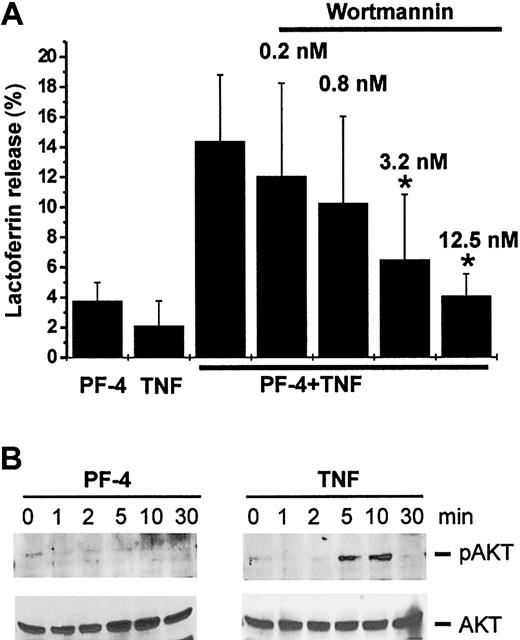
Next, we examined the participation of PI 3-kinase in the signal transduction leading to an exocytosis response. In a first set of experiments, we tested the effect of wortmannin, an inhibitor of the kinase, on PF-4/TNF-induced lactoferrin release. Neutrophils were preincubated in the absence or presence of increasing concentrations of wortmannin, followed by stimulation with PF-4 and TNF. As shown, only the combination of both stimuli was able to induce a relevant exocytosis response (14.4% ± 4.4%). However, preincubation with wortmannin resulted in a dose-dependent reduction of PF-4/TNF-induced exocytosis reaching baseline levels (4.1% ± 1.5% as compared to the effect induced by PF-4 alone 3.8% ± 1.2%) at an inhibitor concentration of 12.5 nM (Figure 4A). Next, a more direct approach was applied for the determination of potential PI 3-kinase activation by probing for phosphorylated AKT kinase (Ser473), a downstream element in the PI 3-kinase pathway (for a review, see Toker27 ). Therefore, neutrophils were stimulated with PF-4 or TNF up to 30 minutes, and phosphorylation of AKT kinase was determined by Western blot analysis with an antiserum specific for the phosphorylated form (Ser473) of the enzyme. Although no AKT kinase phosphorylation exceeding background levels could be observed in PF-4–stimulated neutrophils, the enzyme became significantly phosphorylated in response to TNF (Figure 4B upper panel). Costimulation of cells with a combination of PF-4 and TNF did not change the phosphorylation patterns induced by TNF alone (data not shown). These data clearly demonstrate that TNF-mediated activation of PI 3-kinase is involved in neutrophil exocytosis.
Activation of PI 3-kinase is involved in PF-4/TNF-induced neutrophil exocytosis. (A) Effect of PI 3-kinase inhibitor wortmannin on PF-4/TNF-induced lactoferrin release in human neutrophils. Neutrophils were preincubated for 15 minutes in the presence or absence of increasing concentrations of wortmannin followed by stimulation with PF-4 (4 μM) and TNF (9 ng/mL) alone or in combination for 30 minutes at 37°C. Cells that received stimulus alone and were left without inhibitor served as controls. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds (2.5% ± 0.4%) determined in samples of unstimulated cells run in parallel were subtracted. Data represent mean ± SD of 4 independent experiments, each performed in duplicate. Asterisk (*) indicates significant differences (P < .03) between inhibitor-treated and untreated samples based on the data from 4 individual experiments. (B) Phosphorylation of AKT kinase in response to stimulation with PF-4 or TNF. Neutrophils were stimulated with either PF-4 (4 μM) or TNF (9 ng/mL) for the times indicated. Phosphorylation of AKT kinase was evaluated by Western blot analysis using an antiserum against the Ser473-phosphorylated AKT kinase. Blots were stripped and reprobed with anti-AKT kinase antiserum to confirm equal protein loading. The data from one representative experiment of 3 are given.
Activation of PI 3-kinase is involved in PF-4/TNF-induced neutrophil exocytosis. (A) Effect of PI 3-kinase inhibitor wortmannin on PF-4/TNF-induced lactoferrin release in human neutrophils. Neutrophils were preincubated for 15 minutes in the presence or absence of increasing concentrations of wortmannin followed by stimulation with PF-4 (4 μM) and TNF (9 ng/mL) alone or in combination for 30 minutes at 37°C. Cells that received stimulus alone and were left without inhibitor served as controls. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds (2.5% ± 0.4%) determined in samples of unstimulated cells run in parallel were subtracted. Data represent mean ± SD of 4 independent experiments, each performed in duplicate. Asterisk (*) indicates significant differences (P < .03) between inhibitor-treated and untreated samples based on the data from 4 individual experiments. (B) Phosphorylation of AKT kinase in response to stimulation with PF-4 or TNF. Neutrophils were stimulated with either PF-4 (4 μM) or TNF (9 ng/mL) for the times indicated. Phosphorylation of AKT kinase was evaluated by Western blot analysis using an antiserum against the Ser473-phosphorylated AKT kinase. Blots were stripped and reprobed with anti-AKT kinase antiserum to confirm equal protein loading. The data from one representative experiment of 3 are given.
Phosphorylation of AKT kinase is regulated by p38 MAP kinase
To examine whether signaling pathways leading to the activation of PI 3-kinase and p38 MAP kinase are potentially connected to each other, cross-inhibition experiments were performed where effects of wortmannin on p38 MAP kinase as well as of SB203580 on AKT kinase phosphorylation were analyzed. Neutrophils were preincubated for 15 minutes in the presence of 12.5 nM wortmannin or 10 μM SB203580 or left untreated followed by stimulation with either PF-4 or TNF alone or in combination of both stimuli for 10 minutes. Phosphorylation of p38 MAP kinase as well as activation of PI 3-kinase (as determined by phosphorylated AKT kinase [Ser473]) was assessed by Western blot analysis. As depicted in Figure 5A, preincubation with wortmannin did not alter the phosphorylation state of p38 MAP kinase in stimulated cells, indicating that p38 MAP kinase phosphorylation is not regulated by PI 3-kinase. An effect of SB203580 on p38 MAP kinase could not be anticipated because this inhibitor blocks p38 MAP kinase itself (by binding to its ATP-binding pocket) and not an upstream kinase responsible for the phosphorylation of the enzyme.28 As expected, treatment of neutrophils with wortmannin led to a dramatic inhibition of TNF and TNF/PF-4–induced phosphorylation of AKT kinase (Figure 5B). Interestingly, preincubation of cells with the p38 MAP kinase inhibitor SB203580 also resulted in a complete abrogation of the phosphorylation of AKT kinase at Ser473 (Figure 5B). These observations provide evidence that AKT kinase phosphorylation is directly or indirectly under the regulatory control of p38 MAP kinase.
Effect of wortmannin and SB203580 on the stimulus-induced phosphorylation of p38 MAP kinase and AKT kinase. Neutrophils were preincubated for 15 minutes in the absence or presence of 12.5 nM wortmannin or 10 μM SB203580, and subsequent stimulations were performed with either PF-4 (4 μM) or TNF (9 ng/mL) alone or a combination of both stimuli for 10 minutes. Phosphorylation of p38 MAP kinase (A) was evaluated by Western blot analysis as described in the legend to Figure 2; phosphorylation of AKT kinase (B) was analyzed by Western blot analysis as described in the legend to Figure 4. The data from one representative experiment of 5 and 4 (panels A and B, respectively) are given.
Effect of wortmannin and SB203580 on the stimulus-induced phosphorylation of p38 MAP kinase and AKT kinase. Neutrophils were preincubated for 15 minutes in the absence or presence of 12.5 nM wortmannin or 10 μM SB203580, and subsequent stimulations were performed with either PF-4 (4 μM) or TNF (9 ng/mL) alone or a combination of both stimuli for 10 minutes. Phosphorylation of p38 MAP kinase (A) was evaluated by Western blot analysis as described in the legend to Figure 2; phosphorylation of AKT kinase (B) was analyzed by Western blot analysis as described in the legend to Figure 4. The data from one representative experiment of 5 and 4 (panels A and B, respectively) are given.
The role of src-kinases in PF-4–mediated neutrophil activation
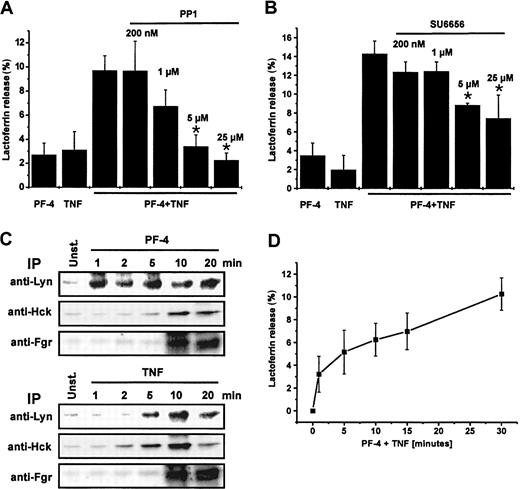
In the last few years great progress has been made in understanding the role of the different tyrosine kinases in the regulation of neutrophil functions.29 Because several recent reports implicate a contribution of members of the src-kinase family in degranulation induced by fMLP in the presence of cytochalasin B, or adhesion-dependent exocytosis responses in neutrophils,24,30 we examined the potential involvement of src-kinases in PF-4/TNF-mediated exocytosis in suspended neutrophils. Therefore, neutrophils were preincubated for 15 minutes in the presence or absence of increasing concentrations of src-kinase inhibitors PP1 or SU6656 and subsequently stimulated with PF-4 or TNF alone or in combination of both stimuli. Preincubation of cells either with PP1 (inhibitor of Lyn and Hck) or SU6656 (Lyn inhibitor) resulted each in a dose-dependent inhibition of the lactoferrin release induced by PF-4/TNF up to 77% (PP1) or 48% (SU6656), respectively (Figure 6A-B). From these data we conclude that src-kinases, primarily Lyn src-kinase, are also involved in PF-4/TNF–induced lactoferrin exocytosis.
The role of src-kinases in PF-4/TNF-mediated exocytosis. (A) Effect of src-kinase inhibitor PP1 or (B) Lyn inhibitor SU6656 on PF-4/TNF-induced lactoferrin release in human neutrophils. Neutrophils were preincubated for 15 minutes in the presence or absence of increasing concentrations of PP1or SU6656, respectively, followed by stimulation with a combination of PF-4 (4 μM) and TNF (9 ng/mL) for 30 minutes at 37°C. Cells that received stimulus alone and were left without inhibitor served as controls. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds (PP1, 1.5% ± 0.1%; or SU6656, 3.1% ± 1.5%, respectively) determined in samples of unstimulated cells run in parallel were subtracted. Data represent mean ± SD of 4 or 3 independent experiments, each performed in duplicate. Asterisk (*) indicates significant differences (PP1, P < .0005; SU6656, P < .007) between inhibitor-treated and untreated samples based on the data from 4 or 3 individual experiments. (C) Activation of srckinases in PF-4– or TNF-stimulated neutrophils. Neutrophils were stimulated with PF-4 (4 μM) or TNF (9 ng/mL) for the time periods indicated and src-kinases were immunoprecipitated (IP) with anti-Lyn, anti-Hck, or anti-Fgr antibodies, respectively, followed by an in vitro phosphorylation assay using acid-denaturated enolase as exogenous substrate. Enolase phosphorylation was visualized using a PhosphorImager system following separation of proteins by SDS-PAGE. (D) Time course of lactoferrin release in response to PF-4/TNF stimulation. Neutrophils were stimulated with a combination of PF-4 (4 μM) and TNF (9 ng/mL) for the times indicated. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds (1.5% ± 0.1%) determined in samples of unstimulated cells run in parallel were subtracted. Data represent mean ± SD of 6 independent experiments, each performed in duplicate.
The role of src-kinases in PF-4/TNF-mediated exocytosis. (A) Effect of src-kinase inhibitor PP1 or (B) Lyn inhibitor SU6656 on PF-4/TNF-induced lactoferrin release in human neutrophils. Neutrophils were preincubated for 15 minutes in the presence or absence of increasing concentrations of PP1or SU6656, respectively, followed by stimulation with a combination of PF-4 (4 μM) and TNF (9 ng/mL) for 30 minutes at 37°C. Cells that received stimulus alone and were left without inhibitor served as controls. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds (PP1, 1.5% ± 0.1%; or SU6656, 3.1% ± 1.5%, respectively) determined in samples of unstimulated cells run in parallel were subtracted. Data represent mean ± SD of 4 or 3 independent experiments, each performed in duplicate. Asterisk (*) indicates significant differences (PP1, P < .0005; SU6656, P < .007) between inhibitor-treated and untreated samples based on the data from 4 or 3 individual experiments. (C) Activation of srckinases in PF-4– or TNF-stimulated neutrophils. Neutrophils were stimulated with PF-4 (4 μM) or TNF (9 ng/mL) for the time periods indicated and src-kinases were immunoprecipitated (IP) with anti-Lyn, anti-Hck, or anti-Fgr antibodies, respectively, followed by an in vitro phosphorylation assay using acid-denaturated enolase as exogenous substrate. Enolase phosphorylation was visualized using a PhosphorImager system following separation of proteins by SDS-PAGE. (D) Time course of lactoferrin release in response to PF-4/TNF stimulation. Neutrophils were stimulated with a combination of PF-4 (4 μM) and TNF (9 ng/mL) for the times indicated. Lactoferrin release was determined in cell supernatants by the use of a sandwich ELISA system. Assay backgrounds (1.5% ± 0.1%) determined in samples of unstimulated cells run in parallel were subtracted. Data represent mean ± SD of 6 independent experiments, each performed in duplicate.
In a more direct approach activation of specific src-kinases was determined by an in vitro phosphorylation assay using acid-denaturated enolase as exogenous substrate. Neutrophils were stimulated for up to 20 minutes with either 4 μM PF-4 or 9 ng/mL TNF and individual src-kinases were immunoprecipitated. Both stimuli were principally able to activate all 3 src-kinases, but displayed striking differences in time kinetics (Figure 6C). Whereas PF-4 induced a rapid activation of Lyn and was maximal already after 1 minute, TNF-mediated activation of Lyn did not become visible until 5 minutes and peaked at 10 minutes of stimulation. Inversely, TNF activated Hck within 2 minutes, whereas PF-4 required approximately 10 minutes of stimulation. However, no differences between both stimuli were seen in the time course of activation of Fgr.
In a next set of experiments we tried to obtain further information about the functional relevance of the fast activation of Lyn. Therefore, time-course experiments of lactoferrin release in response to PF-4/TNF stimulation were performed. Data represented in Figure 6D demonstrate that measurable amounts of lactoferrin could be detected already within 1 minute on stimulation. From these data we conclude that the beginning of the PF-4/TNF-induced exocytosis paralleled with the activation of Lyn.
Effect of various kinase inhibitors on neutrophil adherence
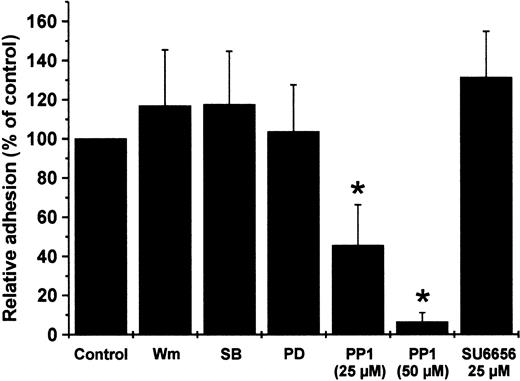
Whereas PF-4–induced exocytosis requires the presence of an appropriate costimulus, neutrophil adherence to endothelial cell layer can be induced by the chemokine alone.13 Consequently, the role of src-kinase and other kinases in PF-4–specific signaling was analyzed by monitoring the effect of various inhibitors including PP1 and SU6656 on neutrophil adherence. In a first approach, neutrophils were preincubated with inhibitors directed against PI 3-kinase (wortmannin), p38 MAP kinase (SB203580), Erk MAP kinases (PD098059), src-kinases Lyn and Hck (PP1), and Lyn (SU6656), and were allowed to adhere to cultured endothelial cells in the presence or absence of PF-4. In accordance with our above results showing that PF-4 does not activate PI 3-kinase, p38 or Erk MAP kinases, treatment of neutrophils with the corresponding inhibitors wortmannin, SB203580, and PD098059 was without effect on PF-4-induced adhesion (Figure 7). In contrast, pretreatment of the cells with src-kinase inhibitor PP1 resulted in a dose-dependent and significant reduction of PF-4–induced neutrophil adherence down to background levels (6.3% ± 4.8%). Interestingly, preincubation with Lyn inhibitor SU6656 was without effect on PF-4–mediated adhesion, indicating that Hck or Fgr may be involved in this process.
Effect of different kinase inhibitors on PF-4–induced neutrophil adhesion to endothelial cells. Neutrophils were pretreated for 20 minutes with wortmannin (Wm; 20 nM), SB203580 (10μM), PD098059 (10 μM), PP1 (25 and 50 μM), SU6656 (25 μM) or without inhibitor and subsequently incubated with 2 μM PF-4 in the presence of a monolayer of cultured endothelial cells. After 20 minutes nonadherent cells were removed from endothelial cells layers and residual neutrophils were determined. The amount of cells in samples receiving PF-4 in the absence of any inhibitor was set at 100% and data were calculated as the percentage of these controls. Data represent mean ± SD of 4 independent experiments, each performed in duplicate. Asterisk (*) indicates significant differences (P < .0005) between inhibitor-treated and untreated samples based on the data from 4 individual experiments.
Effect of different kinase inhibitors on PF-4–induced neutrophil adhesion to endothelial cells. Neutrophils were pretreated for 20 minutes with wortmannin (Wm; 20 nM), SB203580 (10μM), PD098059 (10 μM), PP1 (25 and 50 μM), SU6656 (25 μM) or without inhibitor and subsequently incubated with 2 μM PF-4 in the presence of a monolayer of cultured endothelial cells. After 20 minutes nonadherent cells were removed from endothelial cells layers and residual neutrophils were determined. The amount of cells in samples receiving PF-4 in the absence of any inhibitor was set at 100% and data were calculated as the percentage of these controls. Data represent mean ± SD of 4 independent experiments, each performed in duplicate. Asterisk (*) indicates significant differences (P < .0005) between inhibitor-treated and untreated samples based on the data from 4 individual experiments.
Taken together, our results clearly show that activation of certain src-kinases is involved in PF-4–mediated neutrophil adhesion and exocytosis.
Discussion
During the last few years, signal transduction processes leading to neutrophil activation by chemotactic factors, such as chemokines or formylated peptides, have been under intensive investigation. Within these studies, several signaling elements have been identified, including 7-transmembrane–spanning receptors, heterotrimeric G proteins, calcium transients, or protein kinase C (PKC) isotypes, which appear to be commonly involved in activation processes induced by these factors.5,6,31 Because PF-4 receptors as well as PF-4–mediated biologic activities on neutrophils differ from those of all other chemokines, we assumed that signal transduction pathways initiated by PF-4 may also be different to those known for other chemokines. This view was supported by the results from previous studies showing that PF-4–induced neutrophil activation proceeds independently of heterotrimeric G proteins (F.P., unpublished observations, May 1998) or changes in intracellular free calcium concentrations.12 In the current study we focused on the identification of signaling pathways involved in secondary granule exocytosis and neutrophil adherence induced by PF-4 alone or in combination with TNF.
One of the early intracellular events that occurs during neutrophil activation is the rapid induction of different protein kinase activities, which play essential roles in the regulation of many cellular functions.30,32-39 Among these protein kinases, MAP kinases are highly conserved signaling elements, shown to be involved in the regulation of cell growth,40,41 differentiation,42 and stress responses.43 MAP kinases become activated in human neutrophils by a variety of stimuli, and many neutrophil functions are dependent on p38 MAP kinase activation, for example, apoptosis,25,44 chemotaxis,45 β2-integrin–dependent neutrophil adhesion and adhesion-dependent oxidative burst,37 as well as degranulation.24,46 In suspended neutrophils, neither the platelet-derived CXC chemokine PF-4 nor other potent stimuli like TNF, LPS, or fMLP alone are able to induce a relevant exocytosis response. However, costimulation of PF-4 with any of the latter stimuli results in a massive liberation of secondary granule marker lactoferrin (Figure 3B). Looking for the involvement of p38 MAP kinase in this neutrophil function, we could show that TNF, LPS, as well as fMLP, but not PF-4 induced a significant activation of p38 MAP kinase (Figure 3A). Furthermore, pretreatment of the cells with SB203580 (a p38 MAP kinase inhibitor) significantly reduced lactoferrin release in response to combinations of PF-4 and the mentioned costimuli. SB203580 treatment was without effect on PF-4–induced neutrophil adhesion (Figure 7), which is in line with the lacking capacity of the chemokine to activate the corresponding kinase (Figure 2). Interestingly, the extent of inhibition on neutrophil exocytosis by SB203580 varied among the different stimuli combinations (from 25% to more than 60% inhibition; Figure 3B). In this context it should be noted that in addition to the classical p38 MAP kinase (also known as p38α), a novel isoform of p38 MAP kinase, p38δ has been shown to be present in neutrophils.47 In contrast to p38α, p38δ is not susceptible to treatment with SB203580.43 Therefore, remaining exocytosis in the presence of the inhibitor could be due to activation of p38δ. Whereas LPS stimulation of neutrophils predominantly activates p38α,48 it is presently unknown which of the 2 isoforms becomes activated in neutrophils in response to TNF, fMLP, or anisomycin treatment. However, anisomycin, a pharmacologic activator of p38 MAP kinase, was able to replace physiologic activators TNF, LPS, or fMLP as a costimulus in PF-4–mediated exocytosis. These results taken together allow the conclusion that activation of p38 MAP kinase represents a general and essential costimulatory signal in the induction of an exocytosis response in human neutrophils. Nevertheless, because p38 MAP kinase agonists alone are not able to induce an exocytosis response, an additional signal provided by the PF-4 stimulus must be hypothesized. According to our results, Erk MAP pathway, although clearly activated by TNF, appears to play no or only a minor role in triggering the exocytosis response of neutrophils. This is in line with observations published by Mócsai et al.24 The authors described that Erk MAP kinase activation by fMLP in cytochalasin B–treated neutrophils is not involved in lactoferrin release (degranulation). Otherwise, Erk MAP kinases have been demonstrated to be involved in neutrophil functions like oxidative burst, phagocytosis,39,49 platelet-activating factor (PAF) release,50 p47phox phosphorylation,51 and inhibition of apoptosis.52
As a second signaling element involved in PF-4/TNF-induced exocytosis we could identify PI 3-kinase. PI 3-kinase is activated by a wide variety of hormones, growth factors, and chemoattractants.53-57 In neutrophils, PI 3-kinase is involved in the regulation of cell survival,58 neutrophil adherence to fibrinogen,59,60 cell migration,57 and chemotaxis.61 Susceptibility of PF-4/TNF-induced secondary granule exocytosis toward wortmannin, a specific PI 3-kinase inhibitor, indicates that this kinase is involved in the regulation of this function. By analyzing the phosphorylation status of AKT kinase, a downstream element of the PI 3-kinase signaling pathway, we could directly show that TNF activates this enzyme. PF-4, however, lacks the capacity to activate PI 3-kinase (Figure 4B), and treatment of neutrophils with wortmannin was without effect on PF-4–induced neutrophil adherence (Figure 7). Our findings that PI 3-kinase is not involved in PF-4–mediated adhesion differ from those of other authors describing that fMLP-induced adherence of neutrophils to fibronectin and endothelial cells as well as IL-8–mediated adhesion to plastic surfaces was susceptible to treatment with PI 3-kinase inhibitor wortmannin.59,60,62 These differences might be explained by the different sets of adhesion molecules involved in adherence induced by the individual stimuli. Whereas adhesion mediated by fMLP and IL-8 predominantly activates CD11b/CD18 (MAC-1),13,60,63 which may involve PI 3-kinase, PF-4–induced neutrophil adhesion activates CD11a/CD18 (LFA-1) and CD62L (L-selectin).13
Interestingly, we could show that phosphorylation of AKT kinase was sensitive to treatment with the p38 MAP kinase inhibitor SB203580, whereas p38 MAP kinase phosphorylation was not susceptible to the PI 3-kinase antagonist wortmannin (Figure 5) or src-kinase inhibitor PP1 (data not shown). From these observations we conclude that TNF regulates phosphorylation of AKT kinase at Ser473 through activation of the p38 MAP kinase cascade. Our results are supported by recent findings of Rane et al, who described a similar dependency of AKT kinase phosphorylation on p38 MAP kinase activation in fMLP-, FcγRIIa-, or FcγRIIIb-stimulated neutrophils.26 The authors described that MAPKAP kinase-2, a downstream substrate of p38 MAP kinase, acts as phosphoinositide-dependent kinase 2 (PDK2) for AKT kinase Ser473 phosphorylation.
Having identified 2 signaling elements involved in neutrophil exocytosis, which are activated by TNF, we, moreover, could name src-kinases as potential transducers of PF-4 signals. Activation of the src-kinases in response to different stimuli has been described by several authors.24,64,65 Experiments performed with neutrophils isolated from mice with double deficiency of Fgr and Hck indicate that src-kinases play an essential role in signaling for adhesion-dependent degranulation.30 In addition, investigating fMLP-induced release of primary and secondary granules from suspended neutrophils, Mócsai and colleagues showed that fMLP-induced degranulation is sensitive to PP1, a broad specific src-kinase inhibitor.24
However, it should be mentioned that PF-4 does not induce the intracellular fusion of phagosomes and lysosomes (referred to as “degranulation”), but an exocytosis response (fusion of granules with the plasma membrane) in neutrophils.12 In our experiments we observed that PP1 significantly decreased PF-4/TNF-induced exocytosis as well as PF-4–mediated adherence of neutrophils to endothelial cell layer. Although fMLP-induced degranulation and PF-4/TNF-mediated exocytosis are functionally different, the signaling machinery involved in the control of these functions appears to be at least partially identical. By contrast, a Lyn-specific inhibitor (SU6656) selectively blocked neutrophil exocytosis (Figure 6B) without affecting the adherence response induced by PF-4 (Figure 7). These studies suggested a PF-4–specific activation of Hck or Fgr leading to neutrophil adherence, whereas Lyn, activated by PF-4 or TNF, is responsible for the induction of an exocytosis response.
Surprisingly, a detailed analysis of the individual src-kinase activities following stimulation with PF-4 or TNF revealed the lack of specificity of either stimulus for a certain src-kinase. Furthermore, as the major difference between PF-4 and TNF in their action on src-kinases we could identify stimulus-specific time courses of kinase activation. Whereas PF-4 induces an extremely fast activation of Lyn, TNF acts more rapidly on Hck. Moreover, both stimuli activate Fgr at a rather late time point (10 minutes) to a comparable degree (Figure 6C). Because there exist no mono-specific inhibitors for the different src-kinases, we are not able to fully elucidate the individual contribution of each kinase to the biologic functions of adherence and exocytosis. However, our data suggest that the PF-4–mediated fast activation of Lyn, which occurs simultaneously with the TNF-induced activation of p38 MAP kinase, is essential for the induction of an exocytosis response. Our findings are supported by the observation that in time-course experiments measurable lactoferrin could be detected already within 1 minute on stimulation (Figure 6D). On the other hand, neutrophil adherence, which is not sensitive to SU6656 (Figure 7), may be mediated by Fgr, Hck, or both kinases together. The fact that exocytosis is maximally inhibited by 25 μM PP1, whereas full inhibition of adhesion required slightly higher inhibitor concentrations, strongly suggests that exocytosis and adhesion use overlapping but not identical signaling pathways with different susceptibilities to PP1. On the other hand, PF-4–mediated adhesion depends on at least 2 different adhesion molecules (CD11a/CD18 and L-selectin),13 which may be individually affected by the inhibitor.
In summary, we could show for the first time that at least 3 crucial signaling elements are involved in the induction of neutrophil exocytosis, p38 MAP kinase, PI 3-kinase, and Lyn. Activation of p38 MAP kinase appears to be a general and essential costimulatory signal for PF-4 in the induction of this neutrophil function, and p38 MAP kinase agonists, which are not able to induce a relevant exocytosis response on their own, can act as costimuli for PF-4 in the induction of lactoferrin release. Activation of PI 3-kinase is also induced by TNF and AKT kinase phosphorylation is controlled by p38 MAP kinase. Finally, the rapid activation of Lyn is mediated by PF-4. Based on our results, we hypothesize that cooperation between the different signaling pathways depends not only on the principal activation of the respective elements alone but also on the precise and coordinated time course of this process.
Our present investigations are directed to the identification of signaling elements and mechanisms that are involved in the connection of both pathways.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-08-2802.
Supported in part by Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 415, Projekt B6.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr Martin Ernst (Department of Cell Biology, Research Center Borstel, Germany) for help with statistical analysis.