Abstract
The ability of therapeutic vaccines to generate large numbers of CD8+ T lymphocytes that have specificity for HIV-1 or other virally infected cells has enormous potential clinical value. However, approaches to produce cytotoxic T lymphocytes (CTLs) in vivo via vaccine technology have thus far been disappointing and the ex vivo production of cells for adoptive transfer is labor intensive and expensive. We describe the results of a 2-step antibody-targeting system for the production of CD8+ T lymphocytes specific for HIV-1 and Kaposi sarcoma–associated herpesvirus (KSHV), suitable for use in vivo. In 8 consecutive human leukocyte antigen–A2 (HLA-A2)–positive HIV-1–infected individuals with Kaposi sarcoma, 2 cycles of this system resulted in more than 1 Log increases of specific anti-HIV and anti-KSHV CD8+ lymphocytes. These expanded cells have an effector phenotype that includes the ability to produce interferon-γ and CD45Ra+/CD69+ staining. We have shown that antibody-targeted B cells can function as effective antigen-presenting molecules and lead to sustained specific T-lymphocyte expansion from peripheral blood mononuclear cells (PBMCs) of immunosuppressed individuals. This approach, which offers an easy and effective protocol for the amplification of specific antiviral and antitumor CTLs, may offer significant advances for in vivo T-cell immunotherapeutic protocols.
Introduction
HIV presents unprecedented and formidable challenges to vaccine design1,2 and many consider the goal of sterilizing immunity to be unrealistic.3 As such, the development of more effective treatment strategies and approaches to long-term infection has intensified. In comparison to normal cells, virally infected cells and many tumor cells have recognizable differences in the immunogenic peptides that they display in the grooves of their major histocompatibility complex (MHC) class I molecules. Such immunologic differences and the description of many viral peptides4 may enable effective T-cell–mediated immunotherapy with a limited expanded population of autologous T cells,5 the goal of such therapy being the immune clearance of virally infected cells.
It has been argued that, in HIV, vaccines that induce such cytotoxic T lymphocytes (CTLs) will prove efficacious, provided that responses are of high specificity and magnitude.6 This hypothesis results from the evidence suggesting that following infection, CTLs are able, for some time, to control viral replication. Such data include the following: (1) there is a rise in viral load occurring after CD8-blocking antibodies are infused into macaques infected with simian immunodeficiency virus (SIV)7 ; (2) viral mutation resulting in escape from CD8+ T-cell recognition occurs in HIV and SIV infections8-10 ; (3) long-term nonprogressors maintain good CD8 responses11,12 ; and (4) sex workers who are frequently exposed to HIV and high viral loads seem to be resistant to progressive HIV infection and generate anti-HIV responses through CD8+ T cells.13
The current standard approach to generating antigen-specific CTLs involves their isolation and culturing in vitro using significant quantities of autologous dendritic cells. Oelke et al14 have recently reported that human leukocyte antigen–immunoglobulin (HLA-Ig)–based artificial antigen-presenting cells (aAPCs) support the expansion of both high- and low-affinity CTLs against cytomegalovirus (CMV) and melanoma, respectively. Their system couples a soluble HLA-Ig fusion protein and CD28-specific antibody to beads resulting in antigen-specific CTL expansion in vitro over successive rounds of stimulation. These and other related approaches invariably involve expensive, labor intensive, and cumbersome ex vivo production of large numbers of CTLs for potential adoptive transfer, a process susceptible to contamination at every step.15-19
It has been previously demonstrated that HLA class I/viral peptide complexes targeted to B cells can stimulate specific CTL expansion in vitro.20 Additionally, B cells that are present in large numbers even in immunocompromised patients can act effectively as APCs inducing specific CTL responses in vivo.21,22 Here, using B cells as antigen-presenting cells, we have examined the expansion of specific CTLs against conserved class I–restricted HIV-1 and Kaposi sarcoma–associated herpesvirus (KSHV) epitopes using this 2-step antibody HLA class I/peptide complex delivery system. This procedure, using ex vivo peripheral blood mononuclear cells (PBMCs) obtained from HIV-infected donors should generate large numbers of specific CD8+ T lymphocytes and potentially be a system suited to the generation of such cells in vivo.
Study design
Patients
Between February and July 2003, we recruited 8 consecutive HIV-1–infected individuals with AIDS-related Kaposi sarcoma known to be HLA-A2 positive (Table 1). PBMCs were isolated from 40 mL of venous blood using Ficoll-Histopaque (Sigma, Poole, United Kingdom) density gradient centrifugation. All patients provided written informed consent and the study received ethical approval from the Chelsea and Westminster Hospital ethics committee.
Ex vivo immunization procedure
PBMCs (5 × 106) were incubated with the B9E9 single-chain Fv-streptavidin (scFvSA) fusion protein (10 μg/mL) for one hour at 4°C. The B9E9 scFvSA fusion protein contains the single-chain variable region of the murine IgG2a anti-CD20 murine antibody B9E9 fused to the genomic streptavidin of Streptomyces avidinii. The protein is secreted into the periplasm of genetically engineered Escherichia coli as monomeric subunits (43 400 Daltons) that spontaneously fold into soluble tetramers with a molecular weight of 173 600 Daltons. The 4 antigen-binding and biotin-binding sites of the fusion protein retain the functional capabilities of the parent molecules.24 The fusion protein was a kind gift of Dr J. Schultz (Neorx Corp, Seattle, WA).
After washing in phosphate-buffered saline (PBS), cells were incubated with the biotinylated HLA class I/peptide complex (0.5 μg/mL) for 30 minutes at room temperature. Biotinylated recombinant HLA-A2 class I monomers contained for each patient either the HLA-A*201–restricted Gag peptide (SLYNTVATL) or KSHV glycoprotein B peptide (LMWYEL-SKI25 ) and were obtained from ProImmune (Oxford, United Kingdom). After further washing, cells were placed into 24-well plates at 2.5 × 106 PBMCs and cultured in RPMI with 10% fetal calf serum, L-glutamine, and penicillin/streptomycin (Figure 1). Interleukin-7 (IL-7; R&D Systems, Minneapolis, MN) was added on day 1 at 10 ng/mL, and IL-2 (Chiron, Harefield, United Kingdom) was added at 10 U/mL on day 4, as described previously.26 For further stimulation, fresh PBMCs were obtained from the same patients at weekly intervals and treated as described above. The new autologous “immunized” cells were then mixed with the existing cells at a 1:2 ratio and culturing continued for a further 7 days in 5% CO2 at 37°C.
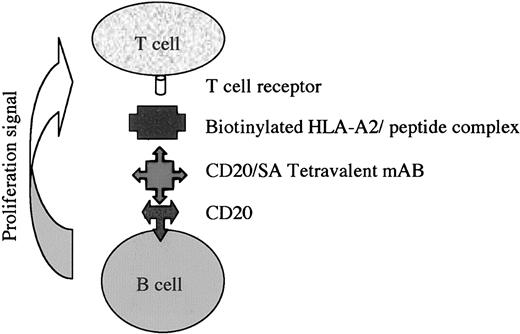
Diagram demonstrating the 2-step antibody-targeted delivery of biotinylated HLA class I/peptide complexes to CD20 on the surface of B cells, as used in these experiments.
Diagram demonstrating the 2-step antibody-targeted delivery of biotinylated HLA class I/peptide complexes to CD20 on the surface of B cells, as used in these experiments.
Flow cytometry and enzyme-linked immunospot (ELIspot) assays
Three-color flow cytometric analysis was performed on 5 × 105 PBMCs removed from the ex vivo culture. These were labeled with phycoerythrin (PE)–conjugated tetramers containing either the HIV-1 gag, KSHV glycoprotein B, or control peptides (ProImmune) and peridinin chlorophyll A protein (PercP)–conjugated anti-CD8 (BD Biosciences, Oxford, United Kingdom). Further phenotyping of cells was performed using fluorescein isothiocyanate (FITC)–conjugated anti-CD27 and anti-CD45Ra (Pharmingen, Oxford, United Kingdom) and allophycocyanin-conjugated anti-CD27 (Pharmingen) and anti-CD69 (Dako, Ely, United Kingdom). A minimum of 100 000 cells were acquired in the live gate and analyzed on a Becton Dickinson FACScaliber using Cell Quest software (Oxford, United Kingdom). Appropriate isotype control antibodies were used in each case.
Interferon γ (IFN-γ) ELIspot assays were carried out as previously described (MABTECH, Stockholm, Sweden)27,28 in triplicate for each patient tested. The HIV-1 gag and KSHV glycoprotein B peptides were incubated at 5 μM with 2 × 105 PBMCs. Results were considered positive if the number of spot-forming cells (SFCs) per million PBMCs in peptide-stimulated wells was 2-fold higher than the number of spots per million PBMCs in control wells and at least 50 spots per million PBMCs were present.
HIV-1–negative and –positive individuals lacking HLA-A2 were used as controls and showed no tetramer-positive staining. HLA class I typing was performed by the Anthony Nolan Trust (Royal Free Hospital, London, United Kingdom) using amplification refractory mutation system–polymerase chain reaction (PCR) with sequence-specific primers.29 Plasma HIV-1 viral loads were determined by the Bayer HIV-1 RNA 3.0 (bDNA) Assay (Newbury, United Kingdom) or by PCR assay (Cobas Amplicor HIV-1 Monitor test version 1.5; Roche Diagnostics, Lewes, United Kingdom) with a lower level of detection of 50 HIV-1 RNA copies/mL. Absolute CD4 and CD8 counts (cells/mm3) were obtained by flow cytometry (Beckman Coulter, Oxford, United Kingdom).
Results and discussion
The lack of an optimal method to produce antigen-specific CTLs has hindered the further development of adoptive immunotherapy. Here, using B cells targeted with HLA class I/peptide complexes as APCs, we show the rapid expansion of specific CD8+ lymphocytes against known HIV and KSHV epitopes using PBMCs from HIV-1–positive individuals. The first step involves the delivery of an anti-CD20 B9E9 fusion protein to the surface of B cells. Step 2 involves the delivery of recombinant biotinylated HLA class I peptide, containing either the HLA-A*201–restricted Gag peptide (SLYNTVATL) or KSHV glycoprotein B peptide (LMWYEL-SKI25 ). Both steps are achieved using whole PBMCs without the need for any further cell separation (Figure 1).
In 8 consecutive patients with differing stages of AIDS-related Kaposi sarcoma (Table 1), 2 successive cycles resulted in the sustained generation of large numbers of CD8+ lymphocytes, specific for HIV or KSHV, in every case (Figure 2A-C). The numbers of KSHV-specific cells increased from 2.1% (interquartile range, 1.2%-4.3%) to 25.7% (16.3%-36.1%), and the numbers of HIV-1–specific cells increased from 1.3% (0.92%-2.0%) to 17.8% (12.0%-42.1%). We have further identified the HIV-positive or KSHV-tetramer positive CD8+ lymphocytes as effector cells, based on their CD69+, CD45Ra+, CD27–, and CD28– phenotype (Figure 2B), and these cells have functional activity, as assessed in IFN-γ ELIspots (Figure 3).
Expansion of tetramer-positive CD8+ T cells. (A) An increase in tetramer-positive CD8+ cells in an individual patient with AIDS-related KS. Tetramer analysis from a single patient demonstrates the increase in specific CTLs to HIV-1 and KSHV over the 2 cycles of targeted therapy in vitro. FL3 (x-axis) is CD8; FL2 (y-axis) corresponds to the tetramer. Top panels show background; middle panels, cycle 1; and bottom panels, cycle 2. (B) The tetramer-positive CD8+ T cells (R2 cycle 2) were further phenotyped and found to express CD45Ra and CD69 with absent CD27 and CD28. (C) The median percentage of tetramer-positive CD8+ cells at background, after one cycle and following 2 cycles of amplification (solid line indicates responses for HIV-1 Gag; dotted line, KSHV glycoprotein B; the median for all 8 patients is shown with the interquartile range).
Expansion of tetramer-positive CD8+ T cells. (A) An increase in tetramer-positive CD8+ cells in an individual patient with AIDS-related KS. Tetramer analysis from a single patient demonstrates the increase in specific CTLs to HIV-1 and KSHV over the 2 cycles of targeted therapy in vitro. FL3 (x-axis) is CD8; FL2 (y-axis) corresponds to the tetramer. Top panels show background; middle panels, cycle 1; and bottom panels, cycle 2. (B) The tetramer-positive CD8+ T cells (R2 cycle 2) were further phenotyped and found to express CD45Ra and CD69 with absent CD27 and CD28. (C) The median percentage of tetramer-positive CD8+ cells at background, after one cycle and following 2 cycles of amplification (solid line indicates responses for HIV-1 Gag; dotted line, KSHV glycoprotein B; the median for all 8 patients is shown with the interquartile range).
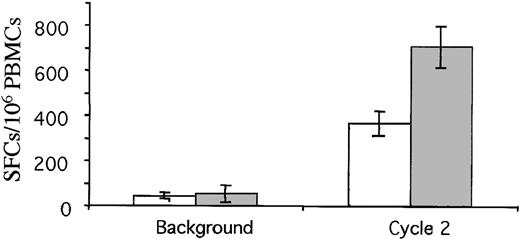
Spot-forming colonies per million PBMCs in IFN-γ ELISpot assays before and after 2 cycles. Median responses to gag (gray bars) and KSHV glycoprotein B (white bars) peptides are shown (± SEM). Responses were specific as demonstrated by a lack of increased responses to other HLA-A2–restricted epitopes.
Spot-forming colonies per million PBMCs in IFN-γ ELISpot assays before and after 2 cycles. Median responses to gag (gray bars) and KSHV glycoprotein B (white bars) peptides are shown (± SEM). Responses were specific as demonstrated by a lack of increased responses to other HLA-A2–restricted epitopes.
The specificity of the T cells produced was further investigated in tetramer and ELIspot analysis using control peptides not corresponding to the specific peptide used in the original monomer. In these control experiments, the number of spot-forming colonies in ELIspots did not rise above baseline levels, supporting the production of specific expansion of T cells recognizing only the HLA class I/peptide complex used. Similarly, staining with HLA-A2/tetramers containing control peptides showed no increase over their prestimulation baseline levels (data not shown)
The responses observed in the ELIspots, greater than a median of 700 IFN-γ ELIspots/106 PBMCs, are 2 to 3 times greater than the proposed peak responses required for an effective HIV vaccine (300 spots/106 PBMCs) based on previous data from nonhuman primates.6
HIV is known to induce a wide array of B-cell dysfunctions including ineffective B-cell costimulatory function with associated low expression of the CD28 ligands, CD80 and CD86.30 However, it is clear from these results that the B cells targeted with the HLA class I/peptide complexes are able to function as effective APCs, suggesting that this 2-step system appears to overcome any effects of reported perturbations in B-cell function.
The efficiency of CTL induction has previously been shown to be related to the stability of the MHC class I/peptide complex on the surface of APCs.25,28,31-33 In this system, we have aimed to optimize the number and stability of expression of the complexes by using fusion proteins and monomers with long half-lives in the context of a high-affinity binding system to a noninternalizing B-cell marker.24 In a number of CTL expansion systems, a relationship between the number of MHC peptide complexes and the level of the T-cell response has been demonstrated34-36 and there are also data suggesting that excess levels of MHC class I/peptide complexes may also result in apoptosis rather than the CTL expansion as shown here.37
In this system, CD20 is present at approximately 87 000 copies per B-cell surface.38 Using the B9E9 scFVSA at concentrations in excess of the affinity constant and with an average of 3.6 biotin-binding sites per antibody, the numbers of immobilized MHC class I/peptide complexes on each B cell should be in the region of 200 000 to 300 000. This is significantly higher than levels produced by peptide pulsing, which results in peptide placement in a maximum of 5000 MHC class I molecules per cell39 of a total number of 100 000 on the surface.40 The functional presence of these recombinant complexes has been shown previously to extend to at least 72 hours41 and is significantly greater than the median half-life of 2.5 to 4 hours of complexes produced with peptide pulsing.42 This enhanced stability combined with their increased number suggests that this method could be expected to enhance the strength and duration of T-cell activation.
This new approach to generating CD8+ T lymphocytes for HIV therapy appears effective in vitro across individuals with a wide range of CD4 lymphopenia and HIV viremia. While to date we have only looked at this preliminary study ex vivo, the technology we describe lends itself to in vivo work, as the B9E9 antibody has already been shown to be safe in patients43 and similar but nontargeted MHC/peptide complexes have been shown to be both immunogenic and nontoxic in mice.44 As we have only been able to show limited expansion to the first round of stimulation (Figure 2C), these data would also argue that clinical trials with this system should allow for at least 2 cycles. In addition to HIV and KSHV, this system that could produce the simple and effective expansion of CTLs in vivo may also be of value in other chronic viral infections treated with adoptive immunotherapy such as in CMV45,46 and Epstein-Barr virus (EBV)19 and also in diseases such as melanoma where currently ex vivo expansion of CTLs and subsequent reinfusion is showing potential.47 In summary we have demonstrated that B cells targeted with HLA class I/peptide complexes can serve as effective antigen-presenting molecules and the CD8+ T lymphocytes expanded have an effector phenotype as demonstrated by interferon-γ production and CD27–/28–/45Ra+/69+ staining. Antiviral and antitumor vaccination procedures based on targeting HLA class I/peptide complexes to B cells in vivo via the antibody delivery system could offer significant advances in both the applicability and effectiveness of such “CTL expanding” approaches.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-09-3023.
Supported in part by Medical Research Council grant no. G84/5631 (J.S.).
A.E. and P.S. have declared a financial interest in Alexis Biotech Ltd., whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to the patients who provided samples.