Abstract
Recipients of HLA-identical stem cell transplants have a poorer transplant outcome if the donor is female rather than male. We analyzed whether pregnancy primes for minor histocompatibility (H) antigens. Peripheral blood mononuclear cells (PBMCs) from healthy multiparous female blood donors were depleted for CD4+, CD14+, CD16+, and CD19+ cells, stained with minor H antigen–specific HLA-A2 tetramers, sorted by fluorescence-activated cell sorting, and tested for cytotoxic activity. Minor H antigens HY-, HA-1–, and HA-2–specific cytotoxic T cells (CD8+, CD45RA–) were present in PBMCs from 4 of 7 female donors up to 22 years after the last delivery. Interestingly, in 2 of the 4 cases microchimerism of the putative immunizing minor H antigen was observed. Thus, pregnancy can lead to alloimmune responses against the infant's paternal minor H antigens. The minor H antigen immunization status of female donors raises important questions for the clinical practice of stem cell transplantation.
Introduction
Pregnancy can lead to alloimmunization and development of erythrocyte- and HLA-specific antibodies.1,2 Stem cell transplantation (SCT), solid organ transplantation, and blood transfusions3-5 immunize for minor histocompatibility (H) antigens. Earlier reports indicated that pregnancy may also induce minor H antigen–specific T cells.6-8 The results may explain the differences in SCT outcome in both the graft-versus-host and host-versus-graft directions. Female-to-male and female-to-female SCTs are more prone to graft-versus-host disease (GVHD) than other donor-recipient combinations.9 Moreover, recipients of stem cells from parous female donors are at increased risk for GVHD compared to recipients of stem cells from nulliparous female donors.10-12 Increased levels of CD8+ T lymphocytes staining with tetrameric HLA class I/HY peptide complexes have been observed in peripheral blood mononuclear cells (PBMCs) of 5 healthy bone marrow donors.13 All 5 donors were women, suggesting the possibility of minor H antigen HY priming during pregnancy. To substantiate this supposition, we collected PBMCs from 7 healthy multiparous female blood donors without a history of blood transfusion. We searched for the presence of circulating minor H antigen–specific T cells with the expected paternal specificity.
Study design
Blood donors
Seven healthy multiparous female blood donors were included in this study after they gave their informed consent. The donors were extensively interviewed for a history of transfusion, tissue or organ transplantation, and pregnancy. Details are given in Table 1. Blood samples were obtained by manual leukapheresis. PBMCs were isolated by Ficoll-Isopaque density gradient centrifugation and stored in liquid nitrogen.
Monoclonal antibodies
Two panels of cell surface molecule-specific antibodies were used. For 3-color flow cytometry analysis: CD8-allophycocyanin (APC; Dako, Glostrup, Denmark), CD45RA-fluorescein isothiocyanate (FITC; Becton Dickinson, San Jose, CA). For 4-color flow cytometry analysis: CD8-phycoerythrin (PE)–Cy7 (Caltag, Burlingame, CA), CD45RA-APC (Serotec, Oxford, United Kingdom) and CD27-FITC (CLB, Amsterdam, The Netherlands).
HLA class I/minor H antigen peptide tetrameric complexes
PE-conjugated HLA-A2/minor H peptide HY (HYA2), HLA-A2/minor H peptide HA-1 (HA-1A2), and HLA-A2/minor H peptide HA-2 (HA-2A2) tetramers were generated as previously described.13
Detection of minor H antigen–specific cytotoxic T lymphocytes
PBMCs of the donors were thawed and resuspended in phosphate-buffered saline (PBS), 10% acid-citrate-dextrose Aqua (ACD-A), 0.5% bovine serum albumin (BSA) buffer for magnetic-activated cell sorting (MACS) depletion using CD4, CD14, CD16, and CD19 magnetic beads according to the supplier's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). The depleted fraction was resuspended in Hanks balanced salt solution (HBSS) without phenol red (Gibco, Paisley, United Kingdom), supplemented with 10% fetal calf serum (FCS) and incubated for 1 hour at 37°C. Single-cell suspensions of 20 to 60 × 106 cells were subsequently stained with PE-conjugated minor H antigen–specific HLA class I tetramers in 100 to 250 μL FACS buffer (HBSS without phenol red, 5% BSA) for 30 minutes at room temperature. Cells were washed and counterstained with a mixture of cell surface antigen-specific antibodies for 20 minutes at 4°C. After washing, enrichment of CD8+, HLA class I/minor H peptide tetramer+ T cells was performed on a FACS-Vantage cell sorter using the “enrich mode,” whereby cells were sorted at 20 000 events/s. The enriched CD8+ tetramer-positive cells were reanalyzed and resorted immediately at a maximum of 10 000 events/s using the more stringent “normal-R” mode. As a negative control, samples were surface-stained with PE-conjugated minor H antigen–specific HLA class I tetramers specific for self minor H antigens.
Culture of minor H antigen–specific cytotoxic T lymphocytes
Double FACS-sorted CD8+ minor H antigen tetramer-positive cells were cultured in 96-well plates (Costar, Corning, NY) at 100 to 1000 cells/well together with 70 000/well irradiated autologous feeder cells. Cell culture medium consisted of Iscove modified Dulbecco medium (IMDM), 10% pooled human serum, 1 μg/mL phytohemagglutinin (PHA), and 20 U/mL interleukin-2 (IL-2) (Cetus, Emeryville, CA). No exogenous specific antigen was added. Fresh medium containing IL-2 was added every 3 days.
Cell-mediated lympholysis assays
Microchimerism analyses
Male microchimerism was detected by real-time polymerase chain reaction (PCR; TaqMan) using Y gene-specific probes. Detection of HA-1 microchimerism was performed by nested PCR using HA-1 allele-specific primer sets, with a sensitivity of 1/105 (B. Wieles, J.P., E.G., manuscript in preparation).
Results and discussion
Seven healthy multiparous women with no history of immunization other than pregnancy were analyzed in this study. They were selected based on HLA-A2 positivity and on delivery of at least one HLA-A2+, HA-1, HA-2, or H-Y disparate child. Table 1 summarizes the relevant information.
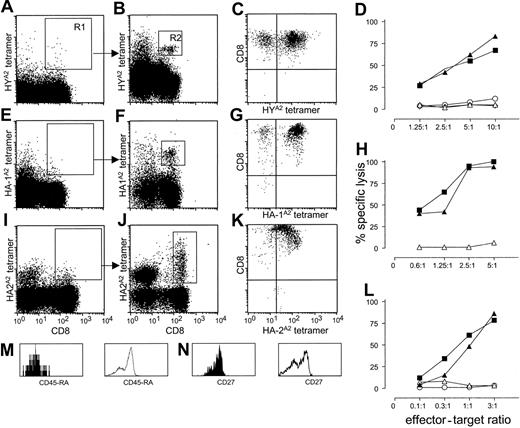
We enriched CD8+ tetramer-positive cells from 300 to 500 × 106 PBMCs by depleting CD4+, CD14+, CD16+, and CD19+ cells. The depleted fraction was stained with CD8, CD45RA, and the various tetramers and enriched by 2 consecutive FACS sorting steps (see “Study design”). Figure 1 illustrates representative analyses from donor nos. 1, 3, and 4. Before FACS, analysis did not reveal a discrete population of tetramer-positive cells (Figure 1A,E,I). After the first sort, the analysis of sorted cells in gate R1 revealed a distinct population of CD8+ cells that stained specifically with the relevant tetramers (Figure 1B,F,J). Some CD8– cells showed nonspecific tetramer staining to a variable degree, depending on the degree of depletion of natural killer (NK) cells by magnetic bead separation. In 4-color FACS analysis, CD8+ tetramer-positive cells (gate R2) stained CD45RA– and CD27+, indicating their “memory” phenotype (filled histograms in Figure 1M-N). Control histograms are given for the CD8+, tetramer– cell population (open histograms in Figure 1M-N). Similarly, we identified HYA2 tetramer-positive cells in donor no. 2 (FACS data not shown). Analyses of donor nos. 5, 6, and 7 revealed neither HY nor HA-1 or HA-2 tetramer-positive cells (Table 1).
Detection and specific lysis of tetramer-positive, CD8+ T cells from PBMCs from multiparous female donors nos. 1, 3, and 4. (A,E,I) Staining of PBMCs with HYA2, HA-1A2, and HA-2A2 tetramers, respectively (y-axis) and CD8 antibody (x-axis) after magnetic bead depletion of CD4+, CD14+, CD16+, and CD19+ cells. (B,F,J) Analysis of cells collected after nonstringent sorting of the rare CD8+, tetramer-positive cells from region R1 for HYA2, HA1A2, and HA-2A2 tetramer, respectively (y-axis), and CD8 (x-axis) staining. In each donor, tetramer-specific cells could be clearly visualized after reanalysis of the cells sorted from region R1 (B,F,J). (M-N) Phenotype analysis of CD8+, HYA2 tetramer-positive cells from donor no. 1 in R2 for the markers CD45RA (M) and CD27 (N; filled histograms); control staining of tetramer-negative cells is represented by open histograms. Bright tetramer staining of polyclonal cultures expanded after double sorting of CD8+ HYA2 and HA-1A2 tetamer-staining cells (C,G). (K) Tetramer staining of a CTL clone generated from HA-2A2 tetramer double-sorted cells. The x-axis represents minor H antigen–specific tetramers; the y-axis represents CD8 staining. (D,H,L) Cytotoxic activity of cultured, double-sorted CTLs. Strong minor H antigen–specific lysis can be observed; x-axis, E/T ratio; y-axis, percent specific lysis. Target cells: ▴, Epstein-Barr virus-/lymphoid cell line (EBV-LCL) positive for the relevant minor H antigen (HY, HA-1, and HA-2 for panels D, H, and L, respectively); ▪, minor H antigen negative EBV-LCL pulsed with the relevant minor H peptide; ▵, EBV-LCL negative for the relevant minor H antigen; ⋄, HLA-mismatched EBV-LCL; ○, K562 cells.
Detection and specific lysis of tetramer-positive, CD8+ T cells from PBMCs from multiparous female donors nos. 1, 3, and 4. (A,E,I) Staining of PBMCs with HYA2, HA-1A2, and HA-2A2 tetramers, respectively (y-axis) and CD8 antibody (x-axis) after magnetic bead depletion of CD4+, CD14+, CD16+, and CD19+ cells. (B,F,J) Analysis of cells collected after nonstringent sorting of the rare CD8+, tetramer-positive cells from region R1 for HYA2, HA1A2, and HA-2A2 tetramer, respectively (y-axis), and CD8 (x-axis) staining. In each donor, tetramer-specific cells could be clearly visualized after reanalysis of the cells sorted from region R1 (B,F,J). (M-N) Phenotype analysis of CD8+, HYA2 tetramer-positive cells from donor no. 1 in R2 for the markers CD45RA (M) and CD27 (N; filled histograms); control staining of tetramer-negative cells is represented by open histograms. Bright tetramer staining of polyclonal cultures expanded after double sorting of CD8+ HYA2 and HA-1A2 tetamer-staining cells (C,G). (K) Tetramer staining of a CTL clone generated from HA-2A2 tetramer double-sorted cells. The x-axis represents minor H antigen–specific tetramers; the y-axis represents CD8 staining. (D,H,L) Cytotoxic activity of cultured, double-sorted CTLs. Strong minor H antigen–specific lysis can be observed; x-axis, E/T ratio; y-axis, percent specific lysis. Target cells: ▴, Epstein-Barr virus-/lymphoid cell line (EBV-LCL) positive for the relevant minor H antigen (HY, HA-1, and HA-2 for panels D, H, and L, respectively); ▪, minor H antigen negative EBV-LCL pulsed with the relevant minor H peptide; ▵, EBV-LCL negative for the relevant minor H antigen; ⋄, HLA-mismatched EBV-LCL; ○, K562 cells.
Next, the functional activities of tetramer-positive cells were analyzed. Sorted HYA2, HA-1A2, and HA-2A2 tetramer-positive cells were expanded nonspecifically, omitting any in vitro minor H antigen–specific stimulation. After 3 weeks, the cultures of donors no. 1 and no. 3 showed high percentages of CD8+ cells brightly staining with the relevant tetramers (Figure 1C,G). Specificity analyses revealed strong minor H antigen–specific lysis (Figure 1D,H) with no lysis against minor H antigen–negative target cells or against the NK-sensitive target cell line K562. T-cell clones generated from polyclonal cultures of donor no. 4 also showed bright staining with HA-2A2 tetramers (Figure 1K) and high levels of HA-2–specific cytotoxic activity at low effector-to-target (E/T) ratios (Figure 1L).
Maintenance of long-term memory may be dependent on antigen-specific triggering. It is known that feto-maternal hemorrhage during pregnancy or at delivery can lead to long-term persisting fetal hematopoietic chimerism.14 We therefore tested PBMCs of the female blood donors for chimeric cells with the relevant paternal minor H genes. Indeed, HY microchimerism in donor no. 1, from whom we isolated HY-specific cytotoxic T lymphocytes (CTLs), and HA-1 microchimerism in donor no. 3, from whom we isolated HA-1–specific CTLs, were observed (Table 1). No chimeric cells were detected in donor no. 2, despite the presence of minor H antigen–specific CTLs. Notably, in the absence of minor H antigen–specific CTLs, no fetal chimerism was observed in the female donor nos. 5, 6, and 7 despite their multiparity. In line with our results, recent murine studies demonstrated that multiparity induces priming to HY antigens.15 The latter murine data, however, do not report on the presence of microchimerism.
Our study unequivocally demonstrates the presence of minor H antigen–specific CTLs in PBMCs from 4 of 7 multiparous healthy female blood donors, who have presumably been exposed to the minor H antigen during pregnancy. The minor H antigen–specific CTLs have a memory phenotype, that is, CD45RA–, are specific for Y chromosome or for autosomally encoded minor H antigens, and remain in the circulation for at least 22 years after delivery. Our findings may explain why recipients of stem cells from parous female donors are at increased risk for GVHD compared to recipients of stem cells from nulliparous female donors.9-11 Likewise, immunized women have a higher risk of transplant rejection.16 The therapeutic implication of our findings is that immunized donors may or may not be better candidates for the ex vivo generation of immunotherapeutic minor H antigen–specific CTLs for the treatment of leukemia relapse after SCT.17
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-05-1625.
Supported in part by a grant from the Leiden University Medical Center.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof C. J. M. Melief and Dr M. Oudshoorn for reading the manuscript.