Abstract
Dendritic cells (DCs) change their antigen-presenting properties during maturation. Immature DCs efficiently capture antigens, but are reported to be impaired in their processing and presenting capacity. Upon an encounter with an inflammatory stimulus, DCs undergo a maturation process that leads to efficient presentation of antigens captured at the time of activation, but precludes processing of antigens encountered at later time points. The mechanisms that underlie these developmental changes are controversial. Thus, it is unclear whether immature DCs can present self antigens, and which are the checkpoints that regulate antigen presentation in immature and mature DCs. We have characterized these mechanisms using DCs derived directly from lymphoid organs. Immature lymphoid organ DCs constitutively presented self peptides bound to major histocompatibility complex class II (MHCII) molecules, but these MHCII-peptide complexes were degraded quickly after their transient expression on the cell surface. During maturation, MHC II endocytosis was down-regulated, so that newly generated MHC II–peptide complexes accumulated on the plasma membrane. Simultaneously, MHC II synthesis was down-regulated, thus preventing the turnover of the MHC II–peptide complexes that accumulated early during maturation. Our results demonstrate that immature DCs constitutively present self antigens in the lymphoid organs and characterize the molecular basis of the capacity of DCs to provide “antigenic memory” in vivo.
Introduction
Naive T lymphocytes circulate through the secondary lymphoid organs, where they use their T-cell receptors to scan for antigenic determinants displayed on the surface of antigen-presenting cells (APCs). The determinants recognized by CD4+ T cells are presented by major histocompatibility complex class II (MHC II) molecules, which are specialized at binding antigenic peptides derived mostly from proteins degraded in endosomal compartments.1 In the absence of foreign antigens, such proteins are either components of the APC itself (ie, endogenous) or exogenous self proteins endocytosed by the APC.2,3 Foreign antigens are incorporated into this pathway by endocytosis, yielding peptides that must compete with the self peptides for binding to MHC II molecules.
Dendritic cells (DCs) are probably the only APCs that can stimulate naive T cells in the secondary lymphoid organs.4 DCs have a complex developmental history. The term “maturation” was originally introduced to refer to the changes undergone by skin Langerhans cells (LCs) (a DC type) during culture in vitro.5 The so-called immature DCs are defined by 3 phenotypic and functional features: (1) they are highly endocytic; (2) they express low levels of MHC II and T-cell costimulatory molecules such as CD40, CD80, and CD86; and (3) they cannot activate naive T cells. During culture, LCs mature to a stage in which they (1) stimulate naive T cells and (2) no longer process and present newly encountered antigens, although they very efficienly present antigens captured before or at the time of receiving the maturation stimulus. Such ability of mature DCs to present antigens they captured in their immature state has been termed antigenic memory.6,7
What are the mechanisms responsible for the developmental control of antigen presentation in DCs? This important aspect of DC biology has been analyzed using DCs generated in vitro from bone marrow (BMDCs), spleen (D1DCs), or blood precursors, but these studies have yielded contradictory results. The results of some experiments suggest that antigen presentation is controlled in DCs at the level of formation of MHC II–peptide complexes8-11 and that immature DCs are inefficient at generating these complexes. However, the results of other experiments support the notion that the control is exerted after the MHC II–peptide complexes have been generated and exposed on the DC surface.9,12-18 An additional complication is that the DCs generated in vitro are at best models for their counterparts in vivo, so it is not clear if any of the mechanisms of control described in those studies apply in vivo. This is an important issue because immature DCs may be responsible for inducing peripheral tolerance,19-21 a role that would obviously require them to present self antigens. Furthermore, the mechanisms that control antigen presentation in vivo are potential targets for the rational design of therapeutic strategies aimed at inducing tolerogenic or immunogenic reactions.
We have analyzed MHC II synthesis, trafficking, peptide loading, and half-life in immature and mature DCs purified from lymphoid organs. We show that the immature lymphoid organ DCs constitutively present self antigens and that the mechanisms that control antigen presentation in DCs in vivo are the rate of MHC II synthesis and the rate of endocytosis of surface MHC II–peptide complexes. These mechanisms of control constitute the biochemical basis of the antigenic memory capacity of DCs in vivo.
Materials and methods
Mice
The mice used were 6- to 8-week-old C57Bl/6 bred at the Walter and Eliza Hall Institute (Parkville, Victoria, Australia) animal breeding facility according to institutional guidelines. Transgenic mice expressing ovalbumin (OVA) under the control of the chicken β-actin promoter (act-OVA mice)22 were provided by Prof Marc J. Jenkins (University of Minnesota Medical School, Minneapolis, MN). Where indicated, mice were injected in the tail vein with phosphate-buffered saline (PBS) or with 3 μg lipopolysaccharide (LPS) (Sigma, St Louis, MO), or with 50 nmol phosphorothioated CpG 1668 (GeneWorks, Adelaide, South Australia, Australia) dissolved in PBS, and were killed 15 hours later.
Reagents
Cycloheximide (CHX) was used at 5 μg/mL and brefeldin A (BFA) was used at 10 μg/mL. Both were from Sigma.
Antibodies and flow cytometry
The antibodies used for preparative or analytical fluorescence-activated cell sorting (FACS) were hamster monoclonal antibody (mAb) N418 (anti-CD11c), rat mAb YTS 169.4 (anti-CD8), rat mAb GK1.5 (anti-CD4), rat mAb NLDC-145 (anti-CD205), rat mAb M5/114 (anti–MHC II), and rat mAb GL1 (anti-CD86). Samples were analyzed by means of a FACS II, FACStarPlus, FACScan, or LSR instrument (Becton Dickinson, San Jose, CA). Preparative FACS was performed by means of a Mo-Flo (Cytomation, Fort Collins, CO) or Diva (Becton Dickinson) high-speed sorter.
Dendritic cell preparation
Spleen and lymph node (LN) DCs were purified as described.23-25 This purification protocol excludes “plasmacytoid” B220+ DCs.26 Separation of the DC subsets was carried out by preparative FACS. The DC culture and the antigen presentation assays were carrried out in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 200 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Pepro Tech, Rocky Hill, NJ).
Antigen presentation to naive OT-II cells and to Ob4 hybridoma T cells
The assays of proliferation of naive I-Ab–restricted, OVA-specific T cells (OT-II cells) were carried out and analyzed as described.25 For the experiment of processing and presentation of exogenous OVA (Figure 1D), freshly isolated or precultured DCs were incubated with 50 μg/mL OVA for 45 minutes at 37°C in culture medium, washed 3 times, and then plated in round-bottom 96-well plates. Carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled OT-II cells were added to the DCs immediately or after 16 hours as indicated. The OT-II cells were purified from transgenic mice and labeled with CFSE as described.27 For the experiments employing fixed DCs (Figure 1E), fixation was perfomed by incubation for 10 minutes at room temperature in 4% paraformaldehyde (PFA) in PBS followed by 3 washes. Fixed cells were plated in round-bottom 96-well plates and then incubated with CFSE-labeled OT-II cells in the presence of 0.1 μg/mL OVA323-339 peptide (Auspep, Melbourne, Victoria, Australia) for 2.5 days. For the experiments employing DCs from act-OVA mice (Figure 2A), the DCs were fixed and then incubated with CFSE-labeled OT-II cells for 2.5 days, or with 105 I-Ab–restricted, OVA-specific Ob4 hybridoma T cells.28 Secretion of interleukin 2 (IL-2) by the Ob4 hybridoma was measured by enzyme-linked immunoabsorbent assay (ELISA).
Splenic DC populations and maturation in culture. (A) Mouse spleens contain 3 populations of CD11c+ DCs that can be distinguished by their expression of CD4 and CD8: CD4+ DCs, CD8+ DCs, and CD4–CD8– DCs. (B) FACS analysis of MHC II and CD86 expression in splenic DCs freshly isolated (f, thin continuous line) or after overnight culture (o/n, thick line). The dashed line corresponds to unlabeled cells (b, background). The histograms correspond to the whole spleen DC preparation; the results were similar for each of the 3 splenic DC subtypes. (C) Immunofluorescence confocal microscopy (ICM) analysis of I-Ab (red) and lysosome-associated membrane protein–1 (Lamp 1) (green) localization in splenic CD8+ DCs freshly isolated (left panels) or after culture for 18 hours (right panels). The dye DAPI (4′,6 diamidino-2-phenylindole) (blue) was included to label the nuclei. Original magnification, × 63. Similar results were obtained with CD4+ DCs and CD4–CD8– DCs (not shown). (D) Left bar: 5 × 103 purified CD8+ DCs were incubated with 50 μg/mL OVA for 45 minutes, washed, and then incubated with naive OVA-specific OT-II cells labeled with CFSE. After 2.5 days in culture, the number of proliferating OT-II cells (propidium iodide [PI]– CFSElow) was determined by FACS analysis. Central bar: after the incubation with OVA, the CD8+ DCs were washed, cultured for 16 hours, and then incubated with OT-II cells for 2.5 days. Right bar: the CD8+ DCs were first cultured for 16 hours and then incubated with OVA for 45 minutes, washed, and incubated with the OT-II cells for 2.5 days. Similar results were obtained with CD8– DCs (not shown).25 (E) Splenic DCs were fixed in PFA immediately after purification (dashed line) or after overnight culture (continuous line) and then incubated with CFSE-labeled OT-II cells in the presence of 0.1 μg/mL OVA323-339 peptide. OT-II proliferation was determined 2.5 days later. Data for experiments in D and E were obtained in duplicate; bars represent the range of the values obtained.
Splenic DC populations and maturation in culture. (A) Mouse spleens contain 3 populations of CD11c+ DCs that can be distinguished by their expression of CD4 and CD8: CD4+ DCs, CD8+ DCs, and CD4–CD8– DCs. (B) FACS analysis of MHC II and CD86 expression in splenic DCs freshly isolated (f, thin continuous line) or after overnight culture (o/n, thick line). The dashed line corresponds to unlabeled cells (b, background). The histograms correspond to the whole spleen DC preparation; the results were similar for each of the 3 splenic DC subtypes. (C) Immunofluorescence confocal microscopy (ICM) analysis of I-Ab (red) and lysosome-associated membrane protein–1 (Lamp 1) (green) localization in splenic CD8+ DCs freshly isolated (left panels) or after culture for 18 hours (right panels). The dye DAPI (4′,6 diamidino-2-phenylindole) (blue) was included to label the nuclei. Original magnification, × 63. Similar results were obtained with CD4+ DCs and CD4–CD8– DCs (not shown). (D) Left bar: 5 × 103 purified CD8+ DCs were incubated with 50 μg/mL OVA for 45 minutes, washed, and then incubated with naive OVA-specific OT-II cells labeled with CFSE. After 2.5 days in culture, the number of proliferating OT-II cells (propidium iodide [PI]– CFSElow) was determined by FACS analysis. Central bar: after the incubation with OVA, the CD8+ DCs were washed, cultured for 16 hours, and then incubated with OT-II cells for 2.5 days. Right bar: the CD8+ DCs were first cultured for 16 hours and then incubated with OVA for 45 minutes, washed, and incubated with the OT-II cells for 2.5 days. Similar results were obtained with CD8– DCs (not shown).25 (E) Splenic DCs were fixed in PFA immediately after purification (dashed line) or after overnight culture (continuous line) and then incubated with CFSE-labeled OT-II cells in the presence of 0.1 μg/mL OVA323-339 peptide. OT-II proliferation was determined 2.5 days later. Data for experiments in D and E were obtained in duplicate; bars represent the range of the values obtained.
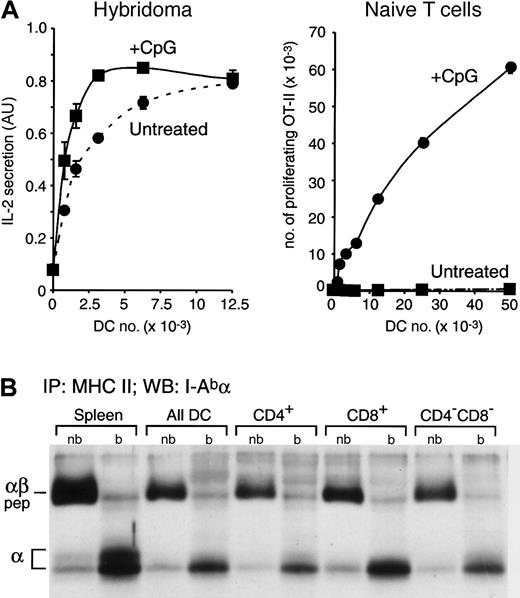
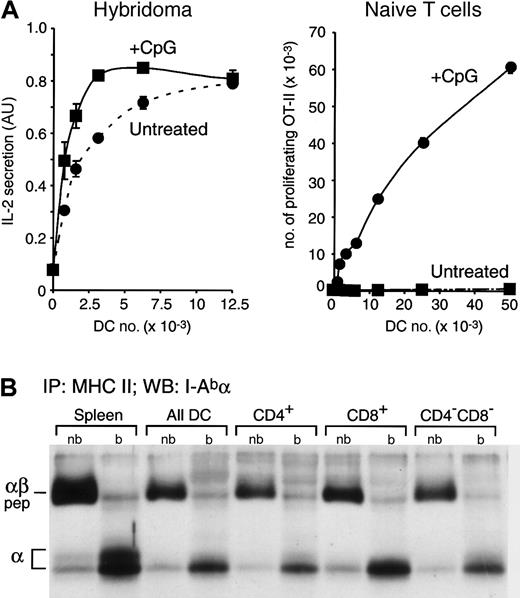
Constitutive presentation of self antigens by immature splenic DCs. (A) Splenic DCs were purified from untreated or CpG-injected OVA+ transgenic mice, which express a membrane-bound form of OVA,22 and then fixed. The indicated number of fixed DCs were incubated with the anti-OVA hybridoma Ob4 (left graph) or with naive OVA-specific OT-II cells (right graph). IL-2 secretion and OT-II proliferation were determined by ELISA 1 day later or by FACS analysis 2.5 days later, respectively. AU indicates arbitrary units. Data for experiments were obtained in duplicate; bars represent the range of the values obtained. (B) MHC II molecules were immunoprecipitated from total splenocytes, a fresh preparation of splenic DCs, and CD4+, CD8+, and CD4–CD8– DCs purified by preparative FACS from the total splenic DC preparation. Immunoprecipitates were loaded in a 12.5% SDS-PAGE without (nb) or after boiling (b) and analyzed by Western blot with a rabbit serum specific for the I-A α-chain. The positions occupied in the blot by the αβ-peptide complexes (αβ-pep) and the free I-Ab α-chain are indicated on the left.
Constitutive presentation of self antigens by immature splenic DCs. (A) Splenic DCs were purified from untreated or CpG-injected OVA+ transgenic mice, which express a membrane-bound form of OVA,22 and then fixed. The indicated number of fixed DCs were incubated with the anti-OVA hybridoma Ob4 (left graph) or with naive OVA-specific OT-II cells (right graph). IL-2 secretion and OT-II proliferation were determined by ELISA 1 day later or by FACS analysis 2.5 days later, respectively. AU indicates arbitrary units. Data for experiments were obtained in duplicate; bars represent the range of the values obtained. (B) MHC II molecules were immunoprecipitated from total splenocytes, a fresh preparation of splenic DCs, and CD4+, CD8+, and CD4–CD8– DCs purified by preparative FACS from the total splenic DC preparation. Immunoprecipitates were loaded in a 12.5% SDS-PAGE without (nb) or after boiling (b) and analyzed by Western blot with a rabbit serum specific for the I-A α-chain. The positions occupied in the blot by the αβ-peptide complexes (αβ-pep) and the free I-Ab α-chain are indicated on the left.
Immunofluorescence confocal microscopy (ICM)
Purified DCs were plated on microscopy coverslips and then fixed, permeabilized, and stained for ICM exactly as described.25
Cell surface biotinylation
DCs were washed 3 times with ice-cold PBS, resuspended in 2 mg/mL EZ-Link sulfo-NHS-skin DC-biotin (Pierce, New York, NY) dissolved in PBS, incubated for 30 minutes in ice, and then washed 3 times with PBS, 10% FCS, and once with RPMI 1640, 10% FCS. Aliquots were frozen immediately or incubated at 37°C in RPMI 1640, 10% FCS, and 200 U/mL GM-CSF and were then spun down and frozen.
Metabolic labeling, immunoprecipitation, SDS-PAGE, and Western blot
Metabolic labeling of cells, immunoprecipitations, and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis were carried out as described.18,29 The mAb used to immunoprecipitate MHC II was N22; mAb Y3 was used for MHC I. The efficiency of incorporation of 35S-Met/Cys in each radiolabeled sample was determined by trifluoroacetic acid (TCA) precipitation of 10 μL cell lysate and counting in a scintillation counter. The amount of immunoprecipitates loaded in the gels was then normalized on the basis of those results. Quantitation of the radioactive polypeptides was performed with a Molecular Dynamics (Amersham, Castle Hill, New South Wales, Australia) phosphorimager. Western blots were performed as described.15,18 Biotinylated polypeptides were detected with the use of avidin–horseradish peroxidase (HRP) (Amersham), and quantitation was performed with a Molecular Dynamics densitometer.
Results
Maturational stage of lymphoid organ DCs and activation in vitro
Mouse spleens contain 3 DC subsets that differ in their surface markers, functional properties, and perhaps hematopoietic lineages (reviewed in Shortman and Liu30 ): CD4+CD8– DCs (“CD4+ DCs” hereafter), CD4–CD8+ DCs (“CD8+ DCs”), and CD4–CD8– DCs (“CD4–CD8– DCs”) (Figure 1A).23 We have recently shown that these 3 DC subsets are phenotypically and functionally immature in the steady state, but can be induced to mature by culture in vitro or by injection of stimulatory compounds in vivo, as illustrated in the following set of experiments.25
First, the freshly isolated spleen DCs expressed moderate levels of MHC II and very low levels of CD86, but during in vitro culture they increased their expression of these 2 markers by 4- to 5-fold and 100-fold, respectively (Figure 1B). Second, the freshly isolated DCs accumulated MHC II in late endosomal/lysosomal Lamp+ compartments, but the MHC II molecules of their cultured counterparts accumulated on the cell surface, whereas the Lamp+ vesicles clustered in a perinuclear region (Figure 1C), a characteristic feature of DC maturation common to in vitro–generated DCs.12,13,15,31 Third, the freshly isolated DCs could process and present the model antigen OVA to naive OT-II T cells, and the presentation still persisted even 16 hours after a brief encounter with OVA (antigenic memory); but cultured DCs were no longer capable of processing and presenting newly encountered antigen (Figure 1D). Fourth, if the freshly isolated DCs were fixed to prevent their maturation in culture, they could not stimulate naive OT-II proliferation, but if they were fixed after culture they could (Figure 1E). These properties define the freshly isolated spleen DCs as immature, and their cultured counterparts as mature.5 Similar phenotypic and functional changes can be induced in vivo by inoculating mice with inflammatory compounds such as LPS or CpG25 (Figure 2), indicating that these changes are the result of a normal program of DC maturation rather than an artifact induced by the in vitro culture.
Immature splenic DCs constitutively present self peptides
We were interested in characterizing the cell biologic mechanisms that regulate MHC II antigen presentation in lymphoid organ DCs. Previous studies of DCs grown in vitro from bone marrow, spleen, or blood precursors have led to 2 contradictory models of regulation. The first proposes that the MHC II molecules of immature DCs are inefficient at acquiring peptide cargo and delivering MHC II–peptide complexes to the cell surface, whereas mature DCs do so efficiently.8-11 The second model suggests that both immature and mature DCs generate MHC II–peptide complexes and deliver them to the plasma membrane, but immature DCs rapidly endocytose and destroy the complexes, whereas in mature DCs the complexes are long-lived on the cell surface.13,15-17 The availability of large numbers of immature (freshly isolated) and mature (cultured) splenic DCs allowed us to address which of these models is at work in DCs generated in vivo.
First we determined whether the freshly isolated DCs expressed MHC II–self peptide complexes. Splenic DCs were isolated from untreated or CpG-injected transgenic mice that express a membrane-bound form of OVA as a model self antigen.22 The DCs were fixed to prevent their maturation in culture, and then incubated with OVA-specific Ob4 hybridoma T cells28 (Figure 2A, left panel). Both DC preparations presented the OVA antigen although, as expected, the DCs from the CpG-injected mice were more efficient because they had been activated in vivo and therefore expressed more MHC II on their surface (Figure 1B).25 To verify that only the DCs from the CpG-injected mice were mature, we assessed the capacity of the same fixed OVA-expressing DCs to induce the proliferation of naive OVA-specific OT-II T cells. Just 0.8 × 103 fixed DCs obtained from CpG-injected mice were sufficient to induce OT-II proliferation, whereas minimal proliferation was observed even with 50 × 103 DCs from untreated mice, demonstrating their immature state (Figure 2A, right panel).
The results shown in Figure 2A demonstrate that immature DCs from mice that express the model self antigen OVA constitutively load their MHC II molecules with OVA-derived peptides and present the complexes on the plasma membrane. However, it could be argued that this particular complex is not representative of the majority of the MHC II molecules. Therefore, we biochemically assessed the structure of the bulk of the MHC II molecules contained in the steady state in immature splenic DCs. In C57Bl/6 mice (I-Ab), the MHC II–peptide complexes are resistant to denaturation in SDS at room temperature, so they run in SDS-PAGE as a complex with a molecular mass (Mr) of approximately 50 kDa.29,32 We immunoprecipitated MHC II from total splenocytes, a preparation of all splenic DCs, and purified CD4+, CD8+, and CD4–CD8– DCs. The immunoprecipitates were loaded in SDS-PAGE with or without boiling, and the position occupied by the MHC II α-chain was then determined by Western blot (Figure 2B). If any of the DC populations failed to generate MHC II–peptide complexes in vivo, their MHC II molecules would not run as SDS-stable complexes. For comparison, we used normal splenocytes (mostly B cells), which constitutively generate MHC II–peptide complexes. As shown previously,18 most of the MHC II α-chains detected in the nonboiled lanes were forming MHC II–peptide complexes (Figure 2B). Indeed, the amount of MHC II–peptide complexes (in the nonboiled immunoprecipitates) relative to the total amount of MHC II α (released in the boiled ones) was comparable in all the DC populations and the splenocytes. We conclude that the bulk of the MHC II molecules of immature DCs constitutively acquire peptides in vivo, just as B cells do. This conclusion was also supported by pulse-chase analysis of MHC II maturation in freshly isolated spleen DCs, which showed that virtually all the newly synthesized MHC II molecules were converted into peptide-loaded complexes.18
Endocytosis and half-life of surface MHC II in immature and mature splenic DCs
In the immature splenic DCs, the majority of the MHC II–peptide complexes were in Lamp+ compartments (Figure 1C). This could be caused by their retention in the endosomal compartments where the MHC II molecules acquire their peptide cargo. Alternatively, it could be that the MHC II–peptide complexes were transiently delivered to the cell surface but were rapidly endocytosed, thus yielding the steady-state distribution observed by microscopy. This second scenario has been shown to occur in both mouse and human DCs grown in vitro.13,15
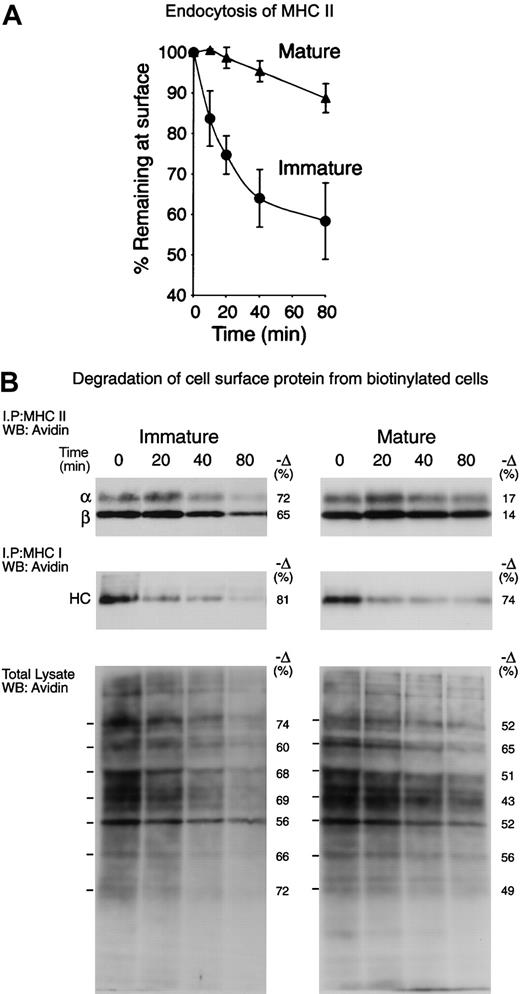
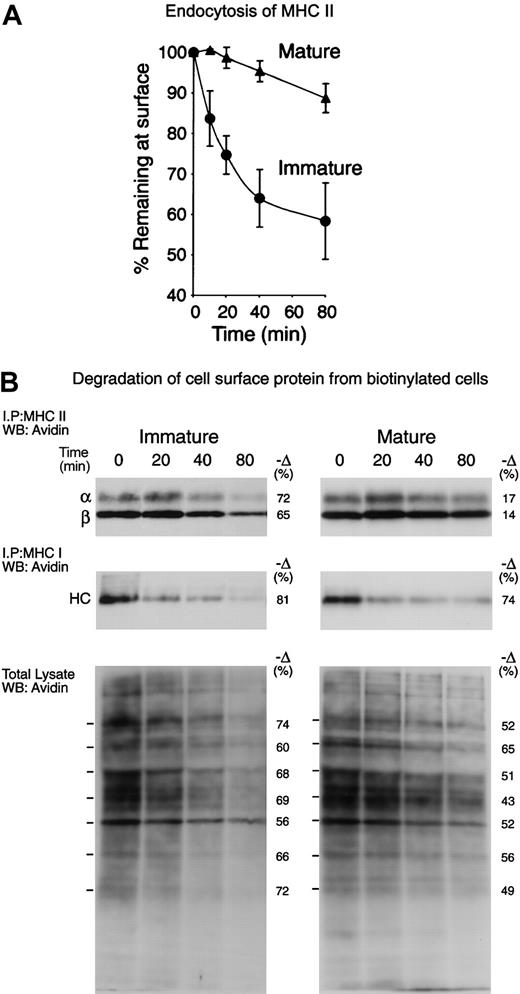
To analyze the MHC II trafficking pathways in immature and mature lymphoid organ DCs, we first assessed by FACS the rate of internalization of a biotinylated mAb bound to surface MHC II (Figure 3A). The immature DCs endocytosed nearly half of their MHC II molecules between 40 and 80 minutes, whereas the mature DCs endocytosed only a small fraction. We then compared the half-life of surface MHC II in the immature and mature DCs. The cells were surface-biotinylated at 4°C and then incubated at 37°C for 0, 20, 40, and 80 minutes. The MHC II molecules were immunoprecipitated from each sample and run in SDS-PAGE. The fraction of biotinylated molecules that remained at each time point was then revealed by Western blot and quantitated (Figure 3B, top panels). Immature DCs degraded approximately 70% of their surface MHC II molecules in 80 minutes, whereas their mature counterparts degraded only 15%. MHC class I molecules were immunoprecipitated sequentially and analyzed similarly, showing that their half-life was very short in both immature and mature DCs (Figure 3B, central panels). Finally, total cell lysates were analyzed to assess the rate of degradation of the bulk of the plasma membrane proteins (Figure 3B, bottom panels). In the immature DCs, the half-life of most surface proteins was comparable to that of MHC I or II, whereas in the mature DCs their half-life was generally longer, though still shorter than the half-life of MHC II. Therefore, surface MHC II was selectively excluded from endocytosis, and subsequent degradation in lysosomal compartments, upon DC maturation. This caused newly arriving MHC II–peptide complexes to accumulate on the plasma membrane of maturing DCs, resulting in increased MHC II levels on the cell surface (Figures 1 and 7). The kinetics of MHC II degradation determined by cell surface biotinylation appeared faster than the rate of endocytosis as determined by FACS (Figure 3A), but it must be noted that the later experiment tracked the internalization of MHC II bound to an antibody, which may impair MHC II endocytosis. Nevertheless, the results of the 2 approaches were consistent with the conclusion that immature DCs endocytosed and then degraded their surface MHC II–peptide complexes in lysosomal compartments much faster than their mature counterparts (Figure 7).
Selective down-regulation of surface MHCII endocytosis and destruction during DC maturation. (A) Freshly isolated (immature) or cultured (mature) splenic DCs were incubated on ice with biotinylated anti–MHC II mAb M5/114, washed, and then incubated at 37°C for 0 to 80 minutes. The amount of MHC II–mAb complexes remaining on the surface was then determined by staining with avidin–fluorescein isothiocyanate (avidin-FITC) and FACS analysis. The graph shows the values of the mean linear fluorescence (MLF) of each sample relative to the values at the 0 time point. The results are the average of 3 independent experiments. (B) Freshly isolated (immature) and cultured (mature) spleen DCs were cell-surface biotinylated at 4°C and then incubated for the times indicated at 37°C. Sequentially immunoprecipitated MHC II (top; the bands correspond to the MHC II α- and β-chains) and MHC I (middle; the band is the MHC I heavy chain) or total cell lysates (bottom) were run in 12.5% SDS-PAGE and analyzed by Western blot with the use of avidin-HRP. Since the cultured cells expressed several times more surface MHC I and II than their fresh counterparts, the exposure times of the pictures shown were adjusted so that the signals of the samples corresponding to the fresh and cultured cells at the 0 time points were comparable. The numbers at the left side of the panels show the relative amount of each indicated polypeptide that was lost between 0 and 80 minutes, as determined by gel densitometry. The results shown are representative of 3 independent experiments
Selective down-regulation of surface MHCII endocytosis and destruction during DC maturation. (A) Freshly isolated (immature) or cultured (mature) splenic DCs were incubated on ice with biotinylated anti–MHC II mAb M5/114, washed, and then incubated at 37°C for 0 to 80 minutes. The amount of MHC II–mAb complexes remaining on the surface was then determined by staining with avidin–fluorescein isothiocyanate (avidin-FITC) and FACS analysis. The graph shows the values of the mean linear fluorescence (MLF) of each sample relative to the values at the 0 time point. The results are the average of 3 independent experiments. (B) Freshly isolated (immature) and cultured (mature) spleen DCs were cell-surface biotinylated at 4°C and then incubated for the times indicated at 37°C. Sequentially immunoprecipitated MHC II (top; the bands correspond to the MHC II α- and β-chains) and MHC I (middle; the band is the MHC I heavy chain) or total cell lysates (bottom) were run in 12.5% SDS-PAGE and analyzed by Western blot with the use of avidin-HRP. Since the cultured cells expressed several times more surface MHC I and II than their fresh counterparts, the exposure times of the pictures shown were adjusted so that the signals of the samples corresponding to the fresh and cultured cells at the 0 time points were comparable. The numbers at the left side of the panels show the relative amount of each indicated polypeptide that was lost between 0 and 80 minutes, as determined by gel densitometry. The results shown are representative of 3 independent experiments
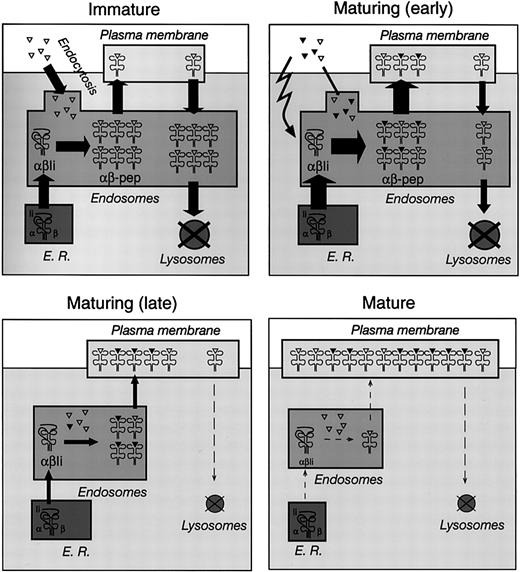
Control of MHC II–peptide endocytosis and MHC II synthesis in immature, maturing, and mature DCs. Schematic representation of MHC II trafficking in immature (top left), maturing (early, top right; late, bottom left), and mature (bottom right) DCs. See “Discussion” for details. The triangles represent self antigens endocytosed by DCs in the steady state (open) or foreign antigens captured in the presence of inflammatory stimuli (closed). The width of the arrows represents the relative flow of MHC II molecules between compartments; the size of the compartments represents the relative amount of MHC II contained in each major subcellular location at each stage of maturation.
Control of MHC II–peptide endocytosis and MHC II synthesis in immature, maturing, and mature DCs. Schematic representation of MHC II trafficking in immature (top left), maturing (early, top right; late, bottom left), and mature (bottom right) DCs. See “Discussion” for details. The triangles represent self antigens endocytosed by DCs in the steady state (open) or foreign antigens captured in the presence of inflammatory stimuli (closed). The width of the arrows represents the relative flow of MHC II molecules between compartments; the size of the compartments represents the relative amount of MHC II contained in each major subcellular location at each stage of maturation.
Mature DCs down-regulate MHC II synthesis
To maintain their steady-state expression of surface MHC II–peptide complexes, splenic DCs must compensate for the loss of the destroyed molecules by maintaining a constant output of newly generated complexes. Conversely, mature DCs must prevent the substitution of their “frozen” surface MHC II by newly arriving molecules (Figure 7). Therefore, we assessed whether the change in MHC II destruction observed during maturation was accompanied by a change in the rate of MHC II synthesis.13-15,33
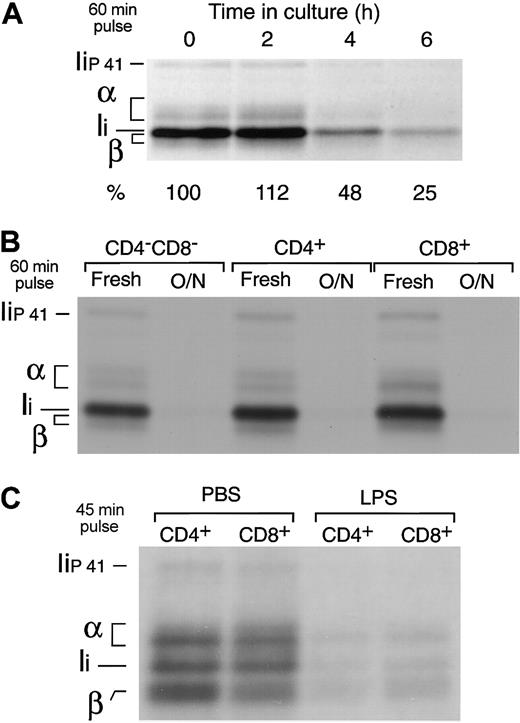
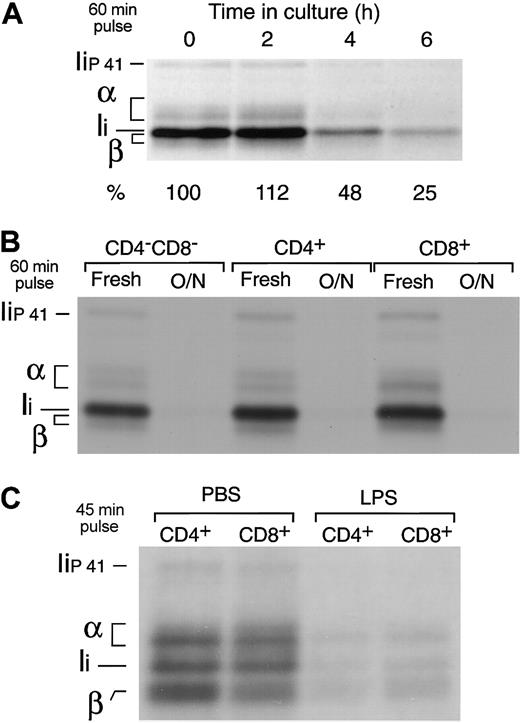
Freshly isolated splenic DCs were put in culture and metabolically labeled for 60 minutes at different time points. The cells were then lysed and their MHC II molecules immunoprecipitated and run in SDS-PAGE. The amount of immunoprecipitate loaded in the gel was normalized for the efficiency of incorporation of 35S-Met/Cys in each sample, as determined by TCA precipitation of the cell lysates. MHC II synthesis increased early during maturation (10% higher after 2 hours), but it decreased to 25% of the original rate 4 hours later (Figure 4A). Indeed, the 3 fully mature splenic DC populations had virtually shut down MHC II synthesis (Figure 4B). In contrast to MHC II, the rate of MHC I synthesis increased in the mature DCs (not shown) (Figure 5), consistent with the observation in the preceding section that MHC I is quickly turned over in both immature and mature DCs (Figure 3B).
Down-regulation of MHC II synthesis during DC maturation. (A) Splenic DCs were metabolically labeled for 60 minutes immediately after purification or after culture for 2 to 6 hours. The cells were lysed and their MHC II molecules immunoprecipitated with mAb N22 and run in 12.5% SDS-PAGE. The amount of immunoprecipitate loaded was normalized for the efficiency of incorporation of radioactivity in each sample, measured by TCA precipitation of the cell lysates. The positions occupied by the α and β subunits of MHC II and the p31 (Ii) and p41 (Iip41) spliced variants of the MHC II chaperone Ii are indicated.1 Note that the MHC II β-chain and Ii overlap in 12.5% SDS-PAGE.34 The numbers at the bottom indicate the relative intensity of the region containing α, β, and Ii in each lane as determined in a phosphorimager. (B) The CD4–CD8–, CD4+, and CD8+ splenic DC subtypes were purified by preparative FACS and analyzed as in panel A immediately after purification or after overnight culture. (C) CD4+ and CD8+ DCs were purified from the spleens of PBS- or LPS-injected mice and metabolically labeled for 45 minutes. The MHC II molecules were immunoprecipitated with mAb N22 and run in 11% SDS-PAGE.
Down-regulation of MHC II synthesis during DC maturation. (A) Splenic DCs were metabolically labeled for 60 minutes immediately after purification or after culture for 2 to 6 hours. The cells were lysed and their MHC II molecules immunoprecipitated with mAb N22 and run in 12.5% SDS-PAGE. The amount of immunoprecipitate loaded was normalized for the efficiency of incorporation of radioactivity in each sample, measured by TCA precipitation of the cell lysates. The positions occupied by the α and β subunits of MHC II and the p31 (Ii) and p41 (Iip41) spliced variants of the MHC II chaperone Ii are indicated.1 Note that the MHC II β-chain and Ii overlap in 12.5% SDS-PAGE.34 The numbers at the bottom indicate the relative intensity of the region containing α, β, and Ii in each lane as determined in a phosphorimager. (B) The CD4–CD8–, CD4+, and CD8+ splenic DC subtypes were purified by preparative FACS and analyzed as in panel A immediately after purification or after overnight culture. (C) CD4+ and CD8+ DCs were purified from the spleens of PBS- or LPS-injected mice and metabolically labeled for 45 minutes. The MHC II molecules were immunoprecipitated with mAb N22 and run in 11% SDS-PAGE.
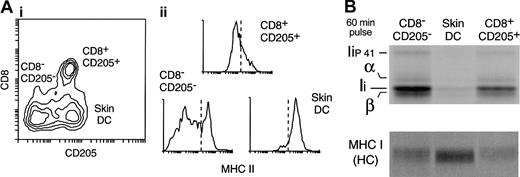
Down-regulation of MHC II synthesis in DCs maturing in vivo in the steady state. (A) DC populations in the subcutaneous LN. A preparation of LN DCs was analyzed by FACS with the use of mAbs for CD11c, CD8, CD205, and MHC II. (i) The contour plot shows the expression of CD8 and CD205 in the CD11c+ cells. Three populations can be distinguished: CD8–CD205– DCs (which include the LN equivalent of the CD4+ and CD4–CD8– splenic DC populations); CD8+CD205+ DCs (the LN equivalent of the splenic CD8+ DCs); and the skin-derived CD8lowCD205+ DCs. (ii) The histograms show the expression level of MHC II in each of the 3 LN DC populations. Note that overlapping populations obscure the distinction between MHC IIlow and MHC IIhigh DCs in the histograms. The dashed line represents an arbitrary division between MHCIIlow and MHChigh expression labels. (B) The 3 LN DC populations were metabolically labeled for 60 minutes immediately after purification. MHC II (top panel) and MHC I (bottom) were immunoprecipitated with mAb N22 and Y3, respectively, and run in a 12.5% SDS-PAGE. The amount of immunoprecipitate loaded was normalized for the efficiency of incorporation of radioactivity in each sample, measured by TCA precipitation of the cell lysates.
Down-regulation of MHC II synthesis in DCs maturing in vivo in the steady state. (A) DC populations in the subcutaneous LN. A preparation of LN DCs was analyzed by FACS with the use of mAbs for CD11c, CD8, CD205, and MHC II. (i) The contour plot shows the expression of CD8 and CD205 in the CD11c+ cells. Three populations can be distinguished: CD8–CD205– DCs (which include the LN equivalent of the CD4+ and CD4–CD8– splenic DC populations); CD8+CD205+ DCs (the LN equivalent of the splenic CD8+ DCs); and the skin-derived CD8lowCD205+ DCs. (ii) The histograms show the expression level of MHC II in each of the 3 LN DC populations. Note that overlapping populations obscure the distinction between MHC IIlow and MHC IIhigh DCs in the histograms. The dashed line represents an arbitrary division between MHCIIlow and MHChigh expression labels. (B) The 3 LN DC populations were metabolically labeled for 60 minutes immediately after purification. MHC II (top panel) and MHC I (bottom) were immunoprecipitated with mAb N22 and Y3, respectively, and run in a 12.5% SDS-PAGE. The amount of immunoprecipitate loaded was normalized for the efficiency of incorporation of radioactivity in each sample, measured by TCA precipitation of the cell lysates.
To verify that down-regulation of MHC II synthesis is a physiological response of DCs to maturation in vivo, we compared the rates of MHC II synthesis in splenic DCs purified from mice injected with PBS or with LPS, which induces DC maturation in vivo.25,35-38 The DCs from both animal groups were labeled for 45 minutes, and their MHC II molecules immunoprecipitated and analyzed by SDS-PAGE. MHC II synthesis decreased by 70% to 85% in the DCs from LPS-treated animals (Figure 4C), whereas MHC I synthesis increased 3-fold (not shown).15
DCs that mature in the steady state in vivo down-regulate MHC II synthesis
To address whether down-regulation of MHC II synthesis occurs in DCs that mature under noninflammatory conditions, we analyzed immature and mature LN DCs. FACS analysis of a subcutaneous LN DC preparation labeled with anti-CD8 and anti-CD205 reveals 3 major groups of CD11c+ DCs (Figure 5A): CD8–CD205– DCs, which include mostly the LN equivalents of the splenic CD4+ and the CD4–CD8– DCs; CD8+CD205+ DCs, which is the LN equivalent of the spleen CD8+ DCs; and CD205+CD8lo DCs, which correspond to skin-derived DCs that have migrated into the subcutaneous LN.25,35,39-41 Of these DC subsets, the skin-derived DC group is the only one with a mature phenotype, as illustrated by its high expression level of MHC II (Figure 5A) (note that the regions containing the skin DCs and the CD205–CD8– DC subset overlap); their incapacity to process and present newly encountered antigens; and their capacity to stimulate naive T-cell proliferation.25 To assess the rate of MHC II synthesis in these 3 DC groups, the cells were purified and metabolically labeled for 60 minutes. Their MHC II molecules were then immunoprecipitated from equivalent amounts of radiolabeled cell lysates (normalized by TCA precipitation) and analyzed by SDS-PAGE. Skin-derived (mature) DCs synthesized much less MHC II than either of the other 2 (mostly immature) LN DC populations (Figure 5B). In contrast, the rate of MHC I synthesis was higher in the skin DCs than in the immature DC groups (Figure 5B).
Most of the MHC II molecules that accumulate on the surface of mature DCs are synthesized de novo
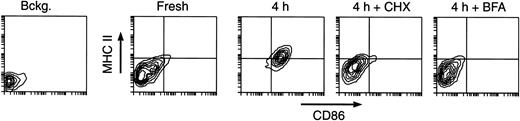
It could be that the MHC II molecules accumulating on the surface of the maturing DCs were either newly synthesized or those contained in the Lamp+ compartments of the immature DCs (Figure 1). We assessed the contribution of newly synthesized molecules by testing the effects of cycloheximide (CHX) (a protein synthesis inhibitor) and brefeldin A (BFA) (an inhibitor of protein export out of the endoplasmic reticulum) on DC maturation. Addition of either drug impaired the increase of MHC II and CD86 expression during in vitro culture (Figure 6). Thus, most MHC II molecules accumulating on the plasma membrane during maturation were derived from de novo synthesis, but as they accumulated on the cell surface, arrival of more molecules was prevented by gradually turning down MHC II synthesis (Figures 4, 5 and 7).
Contribution of de novo synthesis to MHC II expression in maturing DCs. Splenic DCs were cultured for 4 hours in the absence (control) or presence of 5 μg/mL CHX or 10 μg/mL BFA, and analyzed by FACS. The sample labeled “Bckg” was not stained. Only live cells (PI–) were included in the plots. Most of the MHC II molecules accumulating on the surface of maturing DCs are synthesized de novo.
Contribution of de novo synthesis to MHC II expression in maturing DCs. Splenic DCs were cultured for 4 hours in the absence (control) or presence of 5 μg/mL CHX or 10 μg/mL BFA, and analyzed by FACS. The sample labeled “Bckg” was not stained. Only live cells (PI–) were included in the plots. Most of the MHC II molecules accumulating on the surface of maturing DCs are synthesized de novo.
Discussion
The maturational status of DCs has been generally thought to correlate with their anatomic localization.4 Thus, immature DCs can be found in peripheral tissues where they are dedicated to sampling their environment until they receive an inflammatory signal associated with pathogen infection or tissue damage. Upon such signals, DCs become activated, travel to the draining lymphoid organs, mature, and efficiently present the antigens they captured in the peripheral tissue.24,25,41-45 However, DCs are heterogeneous in origin, phenotype, and function,30 and it has become evident that not all the DC types follow this life cycle.25,39,46 We have recently described that in the steady state the only lymphoid organ DC types that are phenotypically and functionally mature are those known to migrate from peripheral tissues, such as the skin, the airways, or the gut, into their corresponding LNs.25 These tissue-derived DCs represent approximately half of the total DCs contained in the steady state in the subcutaneous, mesenteric, mediastinal, and iliac LNs. The remaining 50% of the LN DCs, and virtually all of the splenic DCs, are immature (Figures 1, 2, 5).25 Moreover, these immature DCs can respond to inflammatory stimuli and mature in situ (Figure 2).25,35-38 Similar conclusions have been obtained in studies of human tonsil and spleen DCs.47,48
Such a large cohort of immature lymphoid organ DCs might have 2 major functions: first, they might be responsible for inducing tolerogenic mechanisms in the steady state19,21 ; second, they might play a surveillance role in the lymphoid organs because they have the capacity to respond to pathogens reaching those organs, mature in situ, and then activate naive T cells.25,35-38 Antigen presentation plays a central role in these 2 functions. It is therefore important to characterize how DCs process and present antigen in their immature and mature states in vivo. This study demonstrates that immature DCs constitutively present self antigens in vivo and describes the cell biologic mechanisms that endow DCs with antigenic memory.
Immature DCs constitutively present self antigens
Our results show that the MHC II molecules of the immature lymphoid organ DCs constitutively acquired peptides, thus forming SDS-stable complexes (Figure 2). As in other APCs, in the absence of foreign antigens the peptides contained in these SDS-stable complexes must be derived from endocytosed self proteins either synthesized by the DCs themselves (endogenous) or captured from the surrounding medium (exogenous).2,3 Since immature lymphoid organ DCs expressed relatively high levels of MHC II on their surface, as shown by FACS analysis (Figure 1) and cell surface biotinylation (Figure 3), and virtually all the MHC II molecules expressed by the DCs in the steady state were bound to peptides (Figure 2B), we conclude that immature DCs constitutively deliver to their surface MHC II–self peptide complexes. This conclusion was supported with the analysis of DCs from mice expressing the model antigen OVA. Freshly isolated, fixed immature DCs presented endogenously produced OVA to a T-cell hybridoma even though they were unable to stimulate OVA-specific naive T cells, thus demonstrating their immature state. Similar conclusions have been obtained in studies of antigen presentation of endogenous hen egg lysozyme by splenic DCs or BMDCs.17 Immature BMDCs were also capable of quickly processing and presenting peptides derived from exogenous hen egg lysozyme and pigeon cytochrome C.16,17 All together, these results demonstrate that immature DCs constitutively load their MHC II molecules with antigenic peptides derived from both endogenous and exogenous proteins degraded in their endocytic route, and display the resulting MHC II–peptide complexes on their surface.
The rates of MHC II–peptide endocytosis and MHC II synthesis are coordinately regulated during DC maturation
In immature DCs, the MHC II–peptide complexes delivered to the cell surface had a fast turnover (half-life less than 1 hour) (Figure 3), because they were rapidly endocytosed and degraded in endosomal compartments. This flow of MHC II provoked the steady-state distribution that is characteristic of immature DCs: accumulation in late endosomal/lysosomal compartments (Figures 1 and 7).49 Similar conclusions have been reached in studies of DCs grown in vitro from bone marrow, spleen, and blood precursors.13,15 Since the immature lymphoid organ DCs display considerable endocytic capacity,35 this mechanism might allow these DCs to constantly present an updated sample of the self-antigenic repertoire that reaches the lymphoid organs by itself or is brought by other incoming cells.39,50-54 However, because the MHC II–peptide complexes are quickly turned over, this also implies that each antigenic peptide is presented on the surface at relatively low levels.9,10,16,17
When the freshly isolated DCs became activated by in vitro culture, endocytosis of surface MHC II–peptide complexes was selectively down-regulated, thus protecting the complexes from degradation in lysosomal compartments (Figure 3). The selective retention of MHC II at the surface of mature DCs may be due to their localization in lipid rafts or tetraspanin-rich domains of the plasma membrane.55-59 This caused the accumulation of newly arriving complexes on the plasma membrane of the maturing DCs (Figure 7). The up-regulation of surface MHC II expression in maturing DCs was abrogated by CHX or BFA, demonstrating that most of the molecules deposited on the plasma membrane after activation were synthesized de novo. Unlike the complexes generated in immature DCs, the complexes generated after DC activation may access the plasma membrane through tubulovesicular structures.11,60-64
In step with the changes in MHC II endocytosis, MHC II synthesis increased transiently, but by 18 hours it was nearly shut down.13-15,33 This prevented the substitution of the MHC II–peptide complexes that arrived at the plasma membrane shortly after activation for new complexes (Figure 7). Down-regulation of MHC II synthesis is a physiological response to maturation in vivo, as demonstrated by the low rate of MHC II synthesis in DCs from LPS-injected animals (Figure 4C) or in the mature skin DCs that migrate into the subcutaneous LNs in the steady state (Figure 5). Since the in vivo–matured DCs expressed more MHC II on their surface than their immature counterparts (Figure 5A),25 their lower biosynthetic rate had to be compensated for by a lower turnover rate, consistent with the conclusions of our analysis of spleen-derived DCs.
All the changes in MHC II synthesis, trafficking, and half-life that we described biochemically were fully consistent with the results of the antigen presentation experiments. Antigens captured by immature DCs early during activation were presented for a long time (antigenic memory) (Figure 1D), but once the DCs matured, they were inefficient at presenting newly encountered antigens (Figure 1D)25 even though they still maintained considerable endocytic activity (not shown).65
Our results thus support a model of control of trafficking and expression of MHC II in DCs in vivo based on the kinetics of endocytosis of surface MHC II–peptide complexes rather than on the acquisition of peptide cargo (Figure 7).13,15-17 These results also indicate that the level of MHC II expression can be a misleading parameter when DC maturity is assessed. For example, the immature lymphoid organ DCs express more MHC II on their surface than immature BMDCs and levels similar to those of D1DCs, but the half-life of MHC II in immature D1DCs and BMDCs is several hours,14,15 compared with 1 hour in immature splenic DCs (Figure 3). Similarly, down-regulation of MHC II synthesis during maturation proceeds more slowly, and to a lesser extent, in DCs generated in vitro than in lymphoid organ DCs.13-15 We conclude that the parameter that best defines the maturational state of DCs is the rate of turnover of MHC II–peptide complexes rather than the efficiency of generation of the complexes or the level of their expression on the DC surface.
In conclusion, our study provides support for the hypothesis that immature DCs in the lymphoid organs induce tolerance by constitutively presenting self antigens, and it describes the cell biologic mechanisms that endow mature DCs with their unique capacity to provide a “memory” of the antigens they captured in their immature state.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-08-2729.
Supported by the National Health and Medical Research Council (NHMRC) (J.A.V.); the Cooperative Research Center for Vaccine Technology (N.S.W. and J.A.V.); the Human Frontiers Science Program Organization (J.A.V.); a Melbourne University Research Scholarship (N.S.W.); and a Special Fellowship of the Leukemia and Lymphoma Society (J.A.V.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Marc Jenkins (University of Minnesota Medical School, MN) for the OVA transgenic mice; Dr Lars Karlsson (R. W. Johnson Pharmaceutical Research Institute, San Diego, CA) for the Ob4 hybridoma; and our colleagues Dr Bill Heath and Prof Ken Shortman (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Victoria, Australia) for critically reading the manuscript. We are grateful to the personnel of the FACS and animal breeding facilities at The Walter and Eliza Hall Institute of Medical Research for their excellent technical assistance.

![Figure 1. Splenic DC populations and maturation in culture. (A) Mouse spleens contain 3 populations of CD11c+ DCs that can be distinguished by their expression of CD4 and CD8: CD4+ DCs, CD8+ DCs, and CD4–CD8– DCs. (B) FACS analysis of MHC II and CD86 expression in splenic DCs freshly isolated (f, thin continuous line) or after overnight culture (o/n, thick line). The dashed line corresponds to unlabeled cells (b, background). The histograms correspond to the whole spleen DC preparation; the results were similar for each of the 3 splenic DC subtypes. (C) Immunofluorescence confocal microscopy (ICM) analysis of I-Ab (red) and lysosome-associated membrane protein–1 (Lamp 1) (green) localization in splenic CD8+ DCs freshly isolated (left panels) or after culture for 18 hours (right panels). The dye DAPI (4′,6 diamidino-2-phenylindole) (blue) was included to label the nuclei. Original magnification, × 63. Similar results were obtained with CD4+ DCs and CD4–CD8– DCs (not shown). (D) Left bar: 5 × 103 purified CD8+ DCs were incubated with 50 μg/mL OVA for 45 minutes, washed, and then incubated with naive OVA-specific OT-II cells labeled with CFSE. After 2.5 days in culture, the number of proliferating OT-II cells (propidium iodide [PI]– CFSElow) was determined by FACS analysis. Central bar: after the incubation with OVA, the CD8+ DCs were washed, cultured for 16 hours, and then incubated with OT-II cells for 2.5 days. Right bar: the CD8+ DCs were first cultured for 16 hours and then incubated with OVA for 45 minutes, washed, and incubated with the OT-II cells for 2.5 days. Similar results were obtained with CD8– DCs (not shown).25 (E) Splenic DCs were fixed in PFA immediately after purification (dashed line) or after overnight culture (continuous line) and then incubated with CFSE-labeled OT-II cells in the presence of 0.1 μg/mL OVA323-339 peptide. OT-II proliferation was determined 2.5 days later. Data for experiments in D and E were obtained in duplicate; bars represent the range of the values obtained.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2003-08-2729/6/m_zh80060458360001.jpeg?Expires=1770822138&Signature=ekKS7UoR~vMTnP~htTSIC5t1IZiDTsA1vapmHYtyHU1l45eMIa2BXu1YgsMq1leOuoqURDrJRF-o8pUp5LU5~oPRYzeMoVwShL3Voc9QWXclIkEzEKrCMPtgUCwr2rwE6Mc1JJNmxUk-MO3c~rTsyvEwioPeWsH-2xTEf0WATvkfXQQ2kelpH9YaglO~OEY99IlZiUX0fAxN6DyCeaoSOtNkRrQkMg9xLSba0FWTSyNV0M~YunB5YoJ7e3abqooOk4v~ULkWl9Wx~GZMEYRmFPNOcC4IhRMTu12AOolceoVpHvEgvwSjcqVM6dJwsOKXHmGGPtoyvekhfliBlyNAFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 1. Splenic DC populations and maturation in culture. (A) Mouse spleens contain 3 populations of CD11c+ DCs that can be distinguished by their expression of CD4 and CD8: CD4+ DCs, CD8+ DCs, and CD4–CD8– DCs. (B) FACS analysis of MHC II and CD86 expression in splenic DCs freshly isolated (f, thin continuous line) or after overnight culture (o/n, thick line). The dashed line corresponds to unlabeled cells (b, background). The histograms correspond to the whole spleen DC preparation; the results were similar for each of the 3 splenic DC subtypes. (C) Immunofluorescence confocal microscopy (ICM) analysis of I-Ab (red) and lysosome-associated membrane protein–1 (Lamp 1) (green) localization in splenic CD8+ DCs freshly isolated (left panels) or after culture for 18 hours (right panels). The dye DAPI (4′,6 diamidino-2-phenylindole) (blue) was included to label the nuclei. Original magnification, × 63. Similar results were obtained with CD4+ DCs and CD4–CD8– DCs (not shown). (D) Left bar: 5 × 103 purified CD8+ DCs were incubated with 50 μg/mL OVA for 45 minutes, washed, and then incubated with naive OVA-specific OT-II cells labeled with CFSE. After 2.5 days in culture, the number of proliferating OT-II cells (propidium iodide [PI]– CFSElow) was determined by FACS analysis. Central bar: after the incubation with OVA, the CD8+ DCs were washed, cultured for 16 hours, and then incubated with OT-II cells for 2.5 days. Right bar: the CD8+ DCs were first cultured for 16 hours and then incubated with OVA for 45 minutes, washed, and incubated with the OT-II cells for 2.5 days. Similar results were obtained with CD8– DCs (not shown).25 (E) Splenic DCs were fixed in PFA immediately after purification (dashed line) or after overnight culture (continuous line) and then incubated with CFSE-labeled OT-II cells in the presence of 0.1 μg/mL OVA323-339 peptide. OT-II proliferation was determined 2.5 days later. Data for experiments in D and E were obtained in duplicate; bars represent the range of the values obtained.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2003-08-2729/6/m_zh80060458360001.jpeg?Expires=1770881449&Signature=JTEbgxW0zRwsWy9qbEmejyY-5rpahepaRr2TZWKnNW3yafaCJjmYySBjrEGnclrgvFWvwxe-sZhI51fradn6Bp4~GmhCkbh6z1VYwK8Hs6OeoXXuyRpyP9xdVVXAJwbD~O23fJrLjdLWs3WfyP6PBtHMXnuq3gJwJ5HDmzt4J7kKe94AIIrSoItoMClPa3ZW6t7dYB2Gz0eWSnhYAU2qKU7W-xlBLNdQOIXziFHSV2w8q2UW4OmLHDp6x8r3wbm9d6sW2HmQ0CJwGixUb1lgJ279foKigjYbBys6z~FBT4LaKVJVUMKyzPpEkG~XIO5FCB4ltNMHFdWBWqoO7a3mNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)