Abstract
Although the α2β1 integrin is widely expressed and has been extensively studied, it has not been previously implicated in mast cell biology. We observed that α2 integrin subunit-deficient mice exhibited markedly diminished neutrophil and interleukin-6 responses during Listeria monocytogenes– and zymosan-induced peritonitis. Since exudative neutrophils of wild-type mice expressed little α2β1 integrin, it seemed unlikely that this integrin mediated neutrophil migration directly. Here, we demonstrate constitutive α2β1 integrin expression on peritoneal mast cells. Although α2-null mice contain normal numbers of peritoneal mast cells, these α2-null cells do not support in vivo mast cell–dependent inflammatory responses. We conclude that α2β1 integrin provides a costimulatory function required for mast cell activation and cytokine production in response to infection.
Introduction
The α2β1 integrin, a receptor for collagen, laminin, and other nonmatrix ligands, is expressed on a number of distinct cell types including epithelial cells, endothelial cells, fibroblasts, platelets, and leukocytes.1 On cells of the immune system, the α2β1 integrin is expressed on a subset of activated T lymphocytes (hence the designation very late activation antigen-2), on natural killer (NK) cells (DX5, originally defined as an NK cell–specific marker, was recently shown to recognize the α2β1 integrin), and on neutrophils (polymorphonuclear leukocytes [PMNs]).2,3 Although the role of the α2β1 integrin in immune regulation is poorly understood, accumulating evidence suggests that this integrin may play an important role in leukocyte adhesion and extravasation from the vasculature into peripheral tissues.4-6 Recently, we generated an α2 integrin subunit-deficient mouse model to investigate the role of the α2β1 integrin in normal development and the pathogenesis of disease.7
Here, we report that the α2 integrin–deficient mouse demonstrates a profound and surprising defect in the innate immune response during acute peritonitis. α2 integrin–deficient mice exhibit markedly diminished inflammatory responses to both Listeria monocytogenes, a gram-positive bacterium, and zymosan, a fungal polysaccharide. Although the PMNs of the α2 integrin–deficient mouse lack expression of this integrin, the inflammatory defect in these mice is not due to an inability of the PMNs to extravasate into the peritoneum. Instead, the α2β1 integrin is expressed at high levels on all peritoneal mast cells (PMCs), a cell type required for the induction of the inflammatory response to infection.8,9 We demonstrate that α2β1 integrin expression on the PMC is required for mast cell activation and cytokine release in response to a number of agents in vivo and that α2β1 integrin expression on the PMC is necessary for induction of normal inflammatory responses.
Materials and methods
Mice
α2 integrin–deficient mice on a C57BL/6 × 129/Sv background were used at 6 to 20 weeks of age, with wild-type littermate controls.7 Mice were maintained and bred under specific pathogen-free conditions in the Washington University mouse facility (St Louis, MO). Mast cell–deficient WBB6F1-W/Wv and control WBB6F1-+/+ mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Within individual experiments, mice were appropriately age and sex matched.
Models of peritonitis
Several stimuli were tested for the induction of acute peritoneal inflammation. Listeria strain EGD was stored at mid-log growth as glycerol stocks at -80°C and diluted into pyrogen-free saline for injection into mice. Bacteria were injected at a dose of 5 × 104Listeria/mouse intraperitoneally in 500 μL. Thioglycollate (Sigma-Aldrich, St Louis, MO) was used at a concentration of 4%, injecting 1 mL/mouse intraperitoneally. Zymosan (Sigma-Aldrich) was used at a concentration of 0.4 mg/mL in pyrogen-free saline, injecting 500 μL/mouse. At indicated times after injection, mice were killed and peritoneal exudates were collected by lavage in 10 mL Dulbecco modified Eagle medium (DMEM) or phosphate buffered saline + 10 mM EDTA (ethylenediaminetetraacetic acid) (for zymosan experiments). Cell-free supernatants were stored at -20°C and later used for the determination of interleukin-6 (IL-6) by ELISA (enzyme-linked immunosorbent assay) (BD Biosciences, San Diego, CA). Total cell number was determined for each mouse, and cells were cytospun onto slides and stained with the Hema 3 staining kit (Fisher Scientific, Pittsburgh, PA). The percent PMN was determined by differential cell counting. In experiments with zymosan, PMCs were identified morphologically by their intense basophilic granules on light microscopy, evaluated for evidence of degranulation, and scored as either degranulated or nondegranulated. Statistical analyses were performed using unpaired t tests. In some experiments cells were also analyzed by flow cytometry using the following antibodies (all from BD Biosciences): FITC (fluorescein isothiocyanate)–anti-Gr1 (RB6-8C5), PE (phycoerythrin)–anti–c-kit (2B8), and APC (allophycocyanin)–anti-α2 integrin (DX5).
Generation of bone marrow–derived mast cells
Bone marrow–derived mast cells (BMMCs) were generated as described.10 In brief, bone marrow cells were cultured at 3 to 5 × 105 cells/mL in bone marrow mast cell media (RPMI 1640 containing penicillin 100 U/mL, streptomycin 100 μg/mL, gentamicin 10 μg/mL, l-glutamine 2 mM, nonessential amino acid solution (NEAA) 0.1 mM, 2-mercaptoethanol (2-ME) 50 μM, 10% fetal calf serum, and rIL-3 40 U/mL (PeproTech, Rocky Hill, NJ). Every 7 days nonadherent cells were resuspended in fresh media. Cells were assessed by flow cytometry for the expression of c-kit and α2β1 integrin at 4 to 12 weeks of culture.
Serotonin release assay
PMCs were assessed for their degranulation to Compound 48/80 (Sigma-Aldrich) essentially as described.11 Total peritoneal cells were collected by lavage in DMEM containing 1% bovine serum albumin (BSA), 50 U/mL sodium heparin, and 5 mM EDTA. Cells were washed 3 times in DMEM/1% BSA, adjusted to 5 × 106 cells/mL, and labeled with 2 μCi (0.074 MBq)/mL 3H-serotonin (5-Hydroxy [G-3H] tryptamine, creatinine sulfate; Amersham Biosciences, Piscataway, NJ) for 1 hour at 37°C. Cells were again washed 3 times in DMEM/1% BSA and returned to 5 × 106/mL. Degranulation was carried out in 96-well plates for 1 hour at 37°C, mixing 50 μL of labeled cells with 50 μL of diluted Compound 48/80 (in triplicate). Cell-free supernatants were collected and counted in a scintillation counter (experimental release). Total counts per 50 μL of labeled cells and spontaneously released counts also were determined, to allow the percent serotonin release to be calculated as follows: [(experimental release - spontaneous release)/(total release - spontaneous release)] × 100.
In vitro mast cell adhesion
PMCs, isolated from resident peritoneal exudates using percoll gradient centrifugation (∼85% purity), were used in static adhesion assays.12,13 PMCs (2000 cells/well) were allowed to adhere to coated wells of a 96-well plate for 1 hour at 37°C. Wells were coated with either BSA (0.5 mg/mL), collagen type I (rat tail, 25 μg/mL; BD Biosciences), or fibronectin (bovine plasma, Sigma-Aldrich, 25 μg/mL). Cells were allowed to adhere to BSA or collagen in the presence of 2 mM MgCl2 or 2mM EDTA or to fibronectin in the presence of both 2 mM MgCl2 and 2 mM CaCl2 or 2mM EDTA. Nonadherent cells were removed by washing and the remaining adherent cells quantitated, as described.13
Reconstitution of W/Wv mice with PMCs
In 2 experiments, mast cell–deficient W/Wv mice were reconstituted with mast cells from either α2 integrin–deficient or wild-type littermate controls. In the first of these experiments, PMCs were isolated from resident peritoneal exudates using percoll gradient centrifugation (∼50% purity). In the second experiment, PMCs were isolated from resident peritoneal exudates using a positive selection strategy as follows. Macrophages were removed by a 1 hour adherence to tissue culture plastic at 37°C. Remaining cells were treated with Fc receptor block (2.4G2, 10 μg/mL; BD Biosciences) and subsequently stained with biotin–anti–c-kit (2B8, 10 μg/mL; BD Biosciences). Cells were then labeled with magnetic streptavidin microbeads (Miltenyi Biotec, Auburn, CA) and selected by magnetic separation (MACS; Miltenyi Biotec) according to manufacturer's suggestions. This protocol resulted in more than 95% mast cell purity. In each experiment, a total of 150 000 PMC/mouse were injected intraperitoneally in 1 mL pyrogen-free saline, and mice were used 3 days later for Listeria infection. In both experiments, transplanted mast cells (either α2 integrin wild-type or α2 integrin–deficient) constituted ∼0.2% of total peritoneal cells at day 3 after reconstitution and were clearly identified on cytospin preparations and by flow cytometry. The results of these experiments have been combined, as no differences resulted from the 2 methods of PMC isolation.
Results
α2 integrin–deficient mice display a diminished inflammatory response to Listeria infection
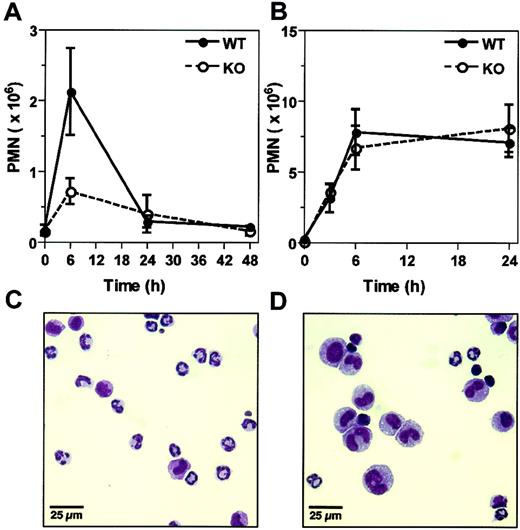
Circulating peripheral blood and splenic PMN counts in unmanipulated wild-type and α2 integrin–deficient mice were comparable. In wild-type mice only about 25% of PMNs in either the peripheral blood or spleen expressed the α2β1 integrin (data not shown). To determine the role of the α2β1 integrin in the acute inflammatory response, PMN influx into the peritoneal cavity in response to Listeria infection was evalutated during the first 48 hours in wild-type and α2 integrin–deficient mice. Wild-type mice displayed a rapid influx of PMNs into the peritoneal cavity, peaking at 6 hours after infection. In contrast, α2 integrin–deficient mice failed to demonstrate this exuberant PMN response, with about 3-fold fewer peritoneal PMNs at 6 hours after infection (Figure 1A,C,D). These initial results suggested that the α2β1 integrin was required for PMN migration into the peritoneum.
α2 integrin–deficient mice display a diminished PMN response to Listeria infection. (A) Wild-type (WT) and α2 integrin–deficient (KO) mice were infected with 5 × 104Listeria intraperitoneally. At indicated times after infection, the absolute peritoneal PMN number was determined. Shown is the combination of 3 experiments (mean ± SEM), with each point representing 4-8 mice. P = .046 at 6 hours after infection. (B) WT and KO mice were injected with thioglycollate intraperitoneally. At indicated times after injection, the absolute peritoneal PMN number was determined. Shown is the combination of 3 experiments (mean ± SEM), with each point representing 5-6 mice (time 0 hours, 3-4 mice). (C,D) Representative cytospin preparations of peritoneal exudates at 6 hours after Listeria infection from (C) wild-type and (D) α2 integrin–deficient mice.
α2 integrin–deficient mice display a diminished PMN response to Listeria infection. (A) Wild-type (WT) and α2 integrin–deficient (KO) mice were infected with 5 × 104Listeria intraperitoneally. At indicated times after infection, the absolute peritoneal PMN number was determined. Shown is the combination of 3 experiments (mean ± SEM), with each point representing 4-8 mice. P = .046 at 6 hours after infection. (B) WT and KO mice were injected with thioglycollate intraperitoneally. At indicated times after injection, the absolute peritoneal PMN number was determined. Shown is the combination of 3 experiments (mean ± SEM), with each point representing 5-6 mice (time 0 hours, 3-4 mice). (C,D) Representative cytospin preparations of peritoneal exudates at 6 hours after Listeria infection from (C) wild-type and (D) α2 integrin–deficient mice.
To further extend these findings, we investigated a second model of acute peritonitis using the nonspecific inflammatory stimulus, thioglycollate. Thioglycollate was injected into the peritoneal cavity of wild-type and α2-null animals, and the PMN influx was evaluated. Surprisingly, both wild-type and α2 integrin–deficient mice displayed equally strong PMN responses to thioglycollate (Figure 1B).
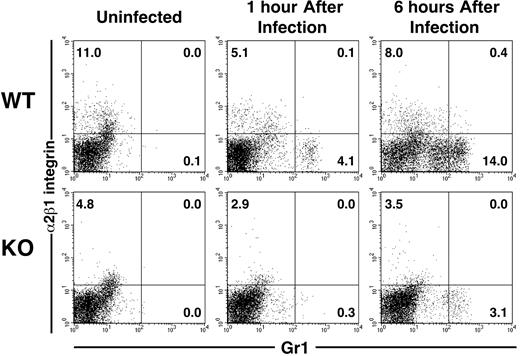
These results suggested that PMNs in the absence of α2β1 integrin were not defective per se. Therefore, we questioned whether the α2β1 integrin was expressed on extravasated PMNs specifically responding to Listeria infection in wild-type mice. Flow cytometric analysis of peritoneal exudates from wild-type Listeria-infected mice revealed that only 3% to 5% of the extravasated PMNs expressed the α2β1 integrin in most animals. In some mice the percentage of extravasated PMNs that expressed the integrin ranged from less than 2% to approximately 15%. Thus, the PMN response was not limited to a subset of PMNs that expressed the integrin (Figure 2).
Flow cytometric analysis of the PMN response to Listeria infection. Wild-type (WT) and α2 integrin–deficient (KO) mice were infected with 5 × 104Listeria intraperitoneally. At indicated times after infection, peritoneal exudate cells were stained with FITC–anti-Gr1 and APC–anti-α2 integrin and assessed by flow cytometry. Numbers represent the percentage of total cells found in each quadrant.
Flow cytometric analysis of the PMN response to Listeria infection. Wild-type (WT) and α2 integrin–deficient (KO) mice were infected with 5 × 104Listeria intraperitoneally. At indicated times after infection, peritoneal exudate cells were stained with FITC–anti-Gr1 and APC–anti-α2 integrin and assessed by flow cytometry. Numbers represent the percentage of total cells found in each quadrant.
Flow cytometric analysis confirmed the diminished PMN response in the α2 integrin–deficient mice. In wild-type mice, PMNs responding to Listeria within the first hour of infection represented mature, Gr1high-staining cells, whereas by 6 hours a large number of responding PMNs were immature, Gr1low-staining cells.14 In α2 integrin–deficient mice, very few PMNs were identified at one hour after infection, and the few PMNs present at 6 hours were mature, Gr1high-staining cells. These data are most consistent with a requirement for the α2β1 integrin during the earliest stage of the inflammatory response to Listeria infection, when bacteria are first detected by resident peritoneal cells, which then call forth inflammatory cells. In other peritoneal infection models, these sentinel cells are the PMCs,8,9 prompting us to question whether PMCs express the α2β1 integrin.
Peritoneal mast cells express the α2β1 integrin
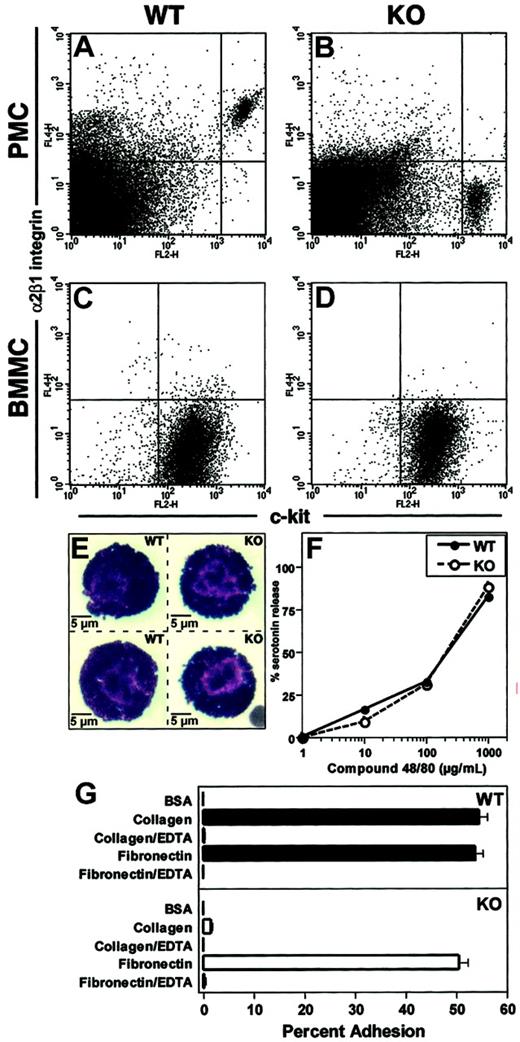
Resident PMCs from wild-type and α2 integrin–deficient mice were identified as c-kithigh-staining cells. Equivalent numbers of PMCs were found in both wild-type and α2 integrin–deficient mice (∼1%-3% of total peritoneal cells, or ∼3 × 104-1.5 × 105 total PMCs per mouse, Figure 3A-B). All PMCs from wild-type mice coexpressed high levels of the α2β1 integrin (in fact, mast cells stained with the highest levels of this integrin of any of the peritoneal leukocytes). PMCs from both wild-type and α2 integrin–deficient mice were of equal size and appeared histologically normal, displaying intense basophilic granules upon Wright-Giemsa staining (Figure 3E). Wild-type and α2 integrin–deficient PMCs also stained similarly with both toluidine blue and chloracetate esterase (data not shown). The degranulation response of PMCs from wild-type and α2 integrin–deficient mice to the nonspecific mast cell degranulating agent Compound 48/80 was similar (Figure 3F). Wild-type PMCs, but not α2 integrin–deficient PMCs, displayed integrin-dependent adhesion to type I collagen (Figure 3G). Both wild-type and α2 integrin–deficient PMCs adhered to fibronectin in a comparable manner. Adhesion of the PMCs to either collagen or fibronectin was not enhanced upon activation with phorbol dibutyrate (data not shown). Interestingly, BMMCs derived from culture in IL-3, more typical of mucosal mast cells,15 did not express the α2β1 integrin, even after as long as 3 months in culture (Figure 3C-D and data not shown).
Peritoneal mast cells express α2β1 integrin. (A,B) Peritoneal exudate cells from unmanipulated wild-type (WT, panel A) and α2 integrin–deficient (KO, panel B) mice were stained with PE–anti–c-kit and APC–anti–α2 integrin and assessed by flow cytometry. Mast cells were identified as c-kithigh-staining cells and represented 1%-3% of resident peritoneal cells in both WT and KO mice. (C,D) BMMC from WT (C) and KO (D) mice at 4 weeks of culture were stained with PE–anti–c-kit and APC–anti–α2 integrin and assessed by flow cytometry. Expression of the α2β1 integrin was not detected. (E) Representative images of PMCs from WT and KO mice stained with Wright-Giemsa stain. Cells were of equal size and contained large numbers of basophilic staining granules. (F) PMCs from WT and KO mice were stimulated with Compound 48/80 at a range of concentrations for 1 hour. Percent serotonin release was calculated (mean ± SEM). (G) PMCs from WT and KO mice were assayed for adhesion to BSA, type I collagen, or fibronectin. Results are presented as means ± SEM.
Peritoneal mast cells express α2β1 integrin. (A,B) Peritoneal exudate cells from unmanipulated wild-type (WT, panel A) and α2 integrin–deficient (KO, panel B) mice were stained with PE–anti–c-kit and APC–anti–α2 integrin and assessed by flow cytometry. Mast cells were identified as c-kithigh-staining cells and represented 1%-3% of resident peritoneal cells in both WT and KO mice. (C,D) BMMC from WT (C) and KO (D) mice at 4 weeks of culture were stained with PE–anti–c-kit and APC–anti–α2 integrin and assessed by flow cytometry. Expression of the α2β1 integrin was not detected. (E) Representative images of PMCs from WT and KO mice stained with Wright-Giemsa stain. Cells were of equal size and contained large numbers of basophilic staining granules. (F) PMCs from WT and KO mice were stimulated with Compound 48/80 at a range of concentrations for 1 hour. Percent serotonin release was calculated (mean ± SEM). (G) PMCs from WT and KO mice were assayed for adhesion to BSA, type I collagen, or fibronectin. Results are presented as means ± SEM.
The inflammatory response to Listeria infection is both mast cell and α2β1 integrin dependent
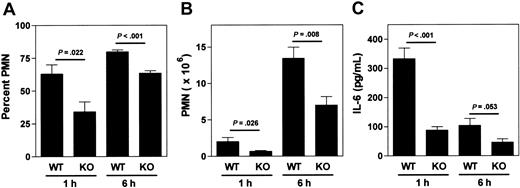
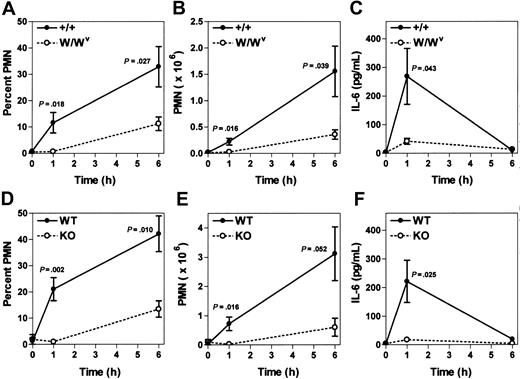
A role for mast cells in the earliest response to Listeria infection has not been previously established. Therefore, mast cell–deficient W/Wv and their control WBB6F1-+/+ (+/+) littermates were infected with Listeria to directly test whether mast cells were required for the normal acute inflammatory response in this model. W/Wv mice showed markedly diminished peritoneal PMN responses at both 1 and 6 hours after infection compared to their control littermates (Figure 4A-B). Mast cell–sufficient +/+ mice, but not W/Wv mice, displayed a sharp spike in peritoneal fluid IL-6 levels at one hour after infection (Figure 4C). These data are highly consistent with findings from other models of acute infectious peritonitis using W/Wv mice in which mast cells are required.8,9 In parallel experiments carried out in α2 integrin–deficient mice and their littermate controls, the early inflammatory response to Listeria infection was the primary focus. The α2β1 integrin was required for both normal peritoneal PMN and IL-6 responses, in a fashion identical to that seen in mast cell–deficient animals (Figure 4D-F). Peritoneal fluid levels of TNF-α were below the limit of detection by our ELISA using the Listeria peritonitis model.
The inflammatory response to Listeria infection is both mast cell– and α2β1 integrin–dependent. (A-C) WBB6F1-+/+ mast cell–sufficient (+/+) and WBB6F1-W/Wv mast cell–deficient (W/Wv) mice were infected with 5 × 104Listeria intraperitoneally. At indicated times after infection, the percentage of PMN, the absolute PMN number, and the IL-6 concentration in peritoneal fluid were determined. Shown is the combination of 2 experiments (mean ± SEM), with each point representing 5-6 mice (time 0 hours, 2 mice). (D-F) Wild-type (WT) and α2 integrin–deficient (KO) mice were infected with 5 × 104Listeria intraperitoneally. At indicated times after infection, the percentage of PMN, the absolute PMN number, and the IL-6 concentration in peritoneal fluid were determined. Shown is the combination of 2 experiments (mean ± SEM), with each point representing 4-5 mice (time 0 hours, 2 mice).
The inflammatory response to Listeria infection is both mast cell– and α2β1 integrin–dependent. (A-C) WBB6F1-+/+ mast cell–sufficient (+/+) and WBB6F1-W/Wv mast cell–deficient (W/Wv) mice were infected with 5 × 104Listeria intraperitoneally. At indicated times after infection, the percentage of PMN, the absolute PMN number, and the IL-6 concentration in peritoneal fluid were determined. Shown is the combination of 2 experiments (mean ± SEM), with each point representing 5-6 mice (time 0 hours, 2 mice). (D-F) Wild-type (WT) and α2 integrin–deficient (KO) mice were infected with 5 × 104Listeria intraperitoneally. At indicated times after infection, the percentage of PMN, the absolute PMN number, and the IL-6 concentration in peritoneal fluid were determined. Shown is the combination of 2 experiments (mean ± SEM), with each point representing 4-5 mice (time 0 hours, 2 mice).
PMC expression of the α2β1 integrin is required for the inflammatory response to Listeria
To conclusively prove that α2β1 integrin expression was specifically required on PMCs for the normal inflammatory response to Listeria, W/Wv mice were selectively reconstituted with purified wild-type or α2 integrin–deficient PMCs. Three days after reconstitution, transferred mast cells were clearly present by flow cytometry (Figure 5A-B). Wild-type PMCs supported an increased inflammatory response to Listeria infection. In contrast, α2 integrin–deficient PMCs were unable to do so (Figure 5C-D). Although the percentage of extravasated PMNs in the peritoneal cavity in response to Listeria by W/Wv mice reconstituted with either wild-type or α2-deficient PMCs was not statistically significant, the absolute number of PMNs responding to Listeria in mast cell–deficient mice reconstituted with wild-type PMCs was statistically different from those reconstituted with α2 integrin–deficient PMCs (P = .039).
Mast cell transfer experiments confirm a requirement for α2β1 integrin on PMCs during the inflammatory response to Listeria. (A,B) Representative flow cytometric profiles of uninfected mast cell–deficient WBB6F1-W/Wv mice reconstituted with wild-type (A) or α2 integrin–deficient (B) PMCs. Reconstituted, uninfected mice contained equivalent numbers of total peritoneal cells (5.6 × 106 [0.21%] for WT reconstituted mice and 5.2 × 106 [0.26%] for KO reconstituted mice) and equivalent percentages of PMCs (approximately 0.2%). (C,D) Mast cell–deficient mice reconstituted with either wild-type (WT) or α2 integrin–deficient (KO) mast cells were infected with 5 × 104Listeria intraperitoneally. At 6 hours after infection, the percentage of PMN and the absolute PMN number were determined. The average total peritoneal cell number in reconstituted, infected mice was as follows: 8.1 × 106 in WT reconstituted mice and 5.6 × 106 in KO reconstituted mice. Shown is a combination of 2 experiments. Uninfected groups each contained 2 mice. Infected groups contained 4-5 mice. Results are presented as means ± SEM.
Mast cell transfer experiments confirm a requirement for α2β1 integrin on PMCs during the inflammatory response to Listeria. (A,B) Representative flow cytometric profiles of uninfected mast cell–deficient WBB6F1-W/Wv mice reconstituted with wild-type (A) or α2 integrin–deficient (B) PMCs. Reconstituted, uninfected mice contained equivalent numbers of total peritoneal cells (5.6 × 106 [0.21%] for WT reconstituted mice and 5.2 × 106 [0.26%] for KO reconstituted mice) and equivalent percentages of PMCs (approximately 0.2%). (C,D) Mast cell–deficient mice reconstituted with either wild-type (WT) or α2 integrin–deficient (KO) mast cells were infected with 5 × 104Listeria intraperitoneally. At 6 hours after infection, the percentage of PMN and the absolute PMN number were determined. The average total peritoneal cell number in reconstituted, infected mice was as follows: 8.1 × 106 in WT reconstituted mice and 5.6 × 106 in KO reconstituted mice. Shown is a combination of 2 experiments. Uninfected groups each contained 2 mice. Infected groups contained 4-5 mice. Results are presented as means ± SEM.
It should be noted that the percentage and number of peritoneal PMNs at 6 hours after infection in mice reconstituted with wild-type PMCs were somewhat lower than in infected +/+ mast cell–sufficient mice (Figure 4A-B). This is likely due to the fact that the reconstituted mice contained only 10% to 20% of the normal number of PMCs by our reconstitution method. The percent and number of peritoneal PMNs at 6 hours after infection in W/Wv mice reconstituted with α2 integrin–deficient PMCs were similar to those levels found in nonreconstituted W/Wv mice (Figure 4A-B).
α2β1 integrin and bacterial burden
The mouse model of Listeria infection is ideal to assess the innate immune response.16 We quantitated Listeria organ burdens in wild-type and α2 integrin–deficient mice and W/Wv mice and their control +/+ littermates. At day 2 after infection, α2 integrin–deficient mice harbored approximately one log more Listeria organisms in their spleen and liver than wild-type controls (Supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, at day 2 after infection, W/Wv and mast cell–sufficient mice harbored identical Listeria burdens in both spleen and liver (Supplemental Figure 1C-D). These data suggested that the increased Listeria burden at day 2 after infection in α2 integrin–deficient mice was not due to an absence of α2β1 integrin expression on mast cells, but instead due to some other defect in innate immunity in these mice. By day 5 after infection, α2 integrin–deficient mice and control mice displayed equivalent Listeria burdens (WT [n = 3]: 4.53 log 10 CFU/spleen, 4.92 log 10 CFU/liver; KO [n = 3]: 4.77 log 10 CFU/spleen, 4.60 4.77 log 10 CFU/liver), suggesting normal adaptive immunity in both sets of mice.
α2β1 integrin is required in a second mast cell–dependent inflammatory response model
In order to confirm the requirement for α2β1 integrin expression during mast cell–dependent inflammatory responses, we treated wild-type and α2 integrin–deficient mice with zymosan, a known inducer of mast cell activation.17 It should be noted, however, that zymosan, unlike Listeria infection, induces significant mast cell–dependent and –independent components of the inflammatory response.17,18 At both 1 and 6 hours after injection, wild-type mice demonstrated a robust acute inflammatory response to zymosan. In contrast, zymosan responses were attenuated in α2 integrin–deficient animals (Figure 6A-B). As in the Listeria model, wild-type mice responded with a burst of IL-6 released into the peritoneal fluid at 1 hour after injection, which was not seen in α2 integrin–deficient mice (Figure 6C).
α2β1 integrin is required during the mast cell–dependent inflammatory response to zymosan. (A-C) Wild-type (WT) and α2 integrin–deficient (KO) mice were injected with zymosan intraperitoneally. At the indicated times after injection, the percent PMN, the absolute PMN number, and the IL-6 concentration in peritoneal fluid were determined. Shown is the combination of 2 experiments (mean ± SEM), with each group containing 5 or 6 mice.
α2β1 integrin is required during the mast cell–dependent inflammatory response to zymosan. (A-C) Wild-type (WT) and α2 integrin–deficient (KO) mice were injected with zymosan intraperitoneally. At the indicated times after injection, the percent PMN, the absolute PMN number, and the IL-6 concentration in peritoneal fluid were determined. Shown is the combination of 2 experiments (mean ± SEM), with each group containing 5 or 6 mice.
α2β1 integrin mediates mast cell degranulation
These in vivo studies suggested that the α2β1 integrin is a critical mediator of mast cell activation. We quantitated the percentage of degranulated mast cells following zymosan-induced inflammation. Sixty-three percent of PMCs in wild-type mice versus 28% of PMCs in α2 integrin–deficient mice showed some evidence of degranulation at 1 hour after zymosan injection (Figure 7A-B). This observation further supports an α2β1 integrin-dependent pathway to mast cell activation in vivo.
Mast cell activation in vivo is α2β1 integrin dependent. (A,B) Wild-type (WT) and α2 integrin–deficient (KO) mice were injected with zymosan intraperitoneally. At 1 hour after injection, peritoneal exudates were analyzed for the percentage of degranulated mast cells. Representative images of WT (scored as degranulated) and KO (scored as nondegranulated) PMCs are shown. Note the abundance of zymosan particles contained in nearby macrophages. Shown is the combination of 2 experiments, with each point representing an individual mouse.
Mast cell activation in vivo is α2β1 integrin dependent. (A,B) Wild-type (WT) and α2 integrin–deficient (KO) mice were injected with zymosan intraperitoneally. At 1 hour after injection, peritoneal exudates were analyzed for the percentage of degranulated mast cells. Representative images of WT (scored as degranulated) and KO (scored as nondegranulated) PMCs are shown. Note the abundance of zymosan particles contained in nearby macrophages. Shown is the combination of 2 experiments, with each point representing an individual mouse.
Discussion
Several important roles have been postulated for the α2β1 integrin during immune responses.4-6,19,20 We now define a requirement for the α2β1 integrin during the inflammatory response of acute peritonitis. α2 integrin–deficient mice demonstrated a markedly diminished inflammatory response to both Listeria infection and zymosan stimulation. Surprisingly, α2β1 integrin expression on the PMN was not required for PMN transmigration or cytokine-driven chemotaxis, as had been expected from earlier reports in the literature. These findings prompted a search for an alternative explanation for the α2β1 integrin–dependent inflammatory response to infectious stimuli.
We discovered high levels of α2β1 integrin expression on murine PMCs, a cell type known to be required for the initiation of inflammatory responses to infection.8,9 Reconstitution of mast cell–deficient W/Wv mice with either wild-type or α2 integrin–deficient mast cells established a specific functional role for α2β1 integrin expression on PMCs in initiating mast cell activation and the acute inflammatory response. Previous studies examining α2β1 integrin expression on primary mast cells and mast cell lines have reported contradictory results, but to our knowledge no report has specifically identified the α2β1 integrin on primary murine connective tissue mast cells such as the PMC.21-24 In contrast to connective tissue mast cells, we also show the absence of α2β1 integrin expression on BMMCs. This, however, is not unexpected as this cell type is more similar to mucosal mast cells, which differ significantly from connective tissue mast cells.15
Both α2 integrin–deficient and W/Wv mast cell–deficient mice displayed similar defects in their acute inflammatory responses to intraperitoneal Listeria infection. α2 integrin–deficient mice harbored approximately one log more Listeria organisms in their spleen and liver at day 2 after infection, while W/Wv and mast cell–sufficient mice were found to harbor identical Listeria burdens in both spleen and liver (Supplemental Figure 1). These results demonstrated that at least in the Listeria model (a facultative intracellular bacterium), the mast cell–dependent responses occurring within the first few hours of infection within the peritoneum did not impact the severity of the eventual infection. This is likely due to the fact that Listeria organisms are known to quickly enter phagocytic cells (particularly macrophages), thus no longer serving as stimuli for mast cell responses. Thus, the increased Listeria burden in α2 integrin–deficient mice was therefore not due to an absence of α2β1 integrin expression on mast cells. Instead, this defect appears to be due to an absence of this integrin's expression on some other cell type important for the control of early Listeria infection.
Mast cells are known to express a variety of integrins required for mast cell development, tissue homing, and responsiveness to stimulation.25-27 PMCs express the αMβ2 integrin (Mac-1) in addition to the α2β1 integrin. αMβ2-deficient mice display diminished homing of mast cells to the peritoneal cavity manifested as decreased absolute numbers of mast cells within the peritoneal cavity and peritoneal wall.28 The RBL-2H3 cell line, a widely used model for rodent mast cells, displays enhanced degranulation to IgE cross-linking upon integrin-mediated adhesion to fibronectin, and anti-integrin antibodies that block fibronectin adhesion can inhibit passive cutaneous anaphylaxis reactions in vivo.29-31 Adhesion to extracellular matrix components also has been shown to provide a costimulatory signal for cytokine production in a human mast cell line.22
In contrast to the mast cell defects in αMβ2 integrin–deficient mice, α2 integrin–deficient PMCs are present in comparable number, size, and granularity to wild-type PMCs. In addition, α2 integrin–deficient PMCs degranulate upon stimulation with the nonspecific degranulating agent Compound 48/80 in a manner similar to wild-type mast cells. Therefore, we favor a model in which α2β1 integrin serves a necessary costimulatory function during the in vivo mast cell response to infection. Several receptors have been identified on mast cells that in combination contribute to this normal response, including toll-like receptors, complement receptors (CD21/CD35), CD19, and CD48.32-37 We have demonstrated both in vivo and in vitro α2β1 integrin–dependent mast cell degranulation and cytokine production in response to infectious stimuli. We hypothesize a costimulatory function for α2β1 integrin on mast cells similar to that reported on T cells during stimulation via the T-cell receptor in the presence of collagen.19,20 Likewise, the α2β1 integrin plays a primary role in platelet adhesion to collagen and a cooperative role with the low-affinity collagen receptor, glycoprotein VI (GPVI), during platelet aggregation and degranulation in response to collagen.7,38 It should be noted that all platelets, all murine natural killer (NK) cells, and all murine PMCs constitutively express the α2β1 integrin. Biologic responses by each of these cell types involve cell-cell or cell-matrix interactions, coreceptor signaling, and ultimate activation leading to degranulation, potentially suggesting a common role for α2β1 integrin ligation in supporting these responses.
At this time, the ligand for α2β1 integrin during the PMC response to infection remains unknown. Collagen has been reported to be a component of the peritoneal stroma,39 although free collagen within the peritoneal cavity seems unlikely. Recently, E-cadherin, an adhesion molecule expressed on epithelial cells and PMCs themselves, has been identified as a ligand for α2β1 integrin.40 Heterotypic mast cell contact with other cells has been reported to influence mast cell responses, leaving this as a possibility.41-44
Our final hypothesis suggests the potential interaction of α2β1 integrin with certain microbial pattern recognition molecules of the innate immune system functioning with immune complexes. Collectins, ficolins, and the C1q complement protein all contain collagen-like sequences and are known to coat the surface of microbes.45 While purely speculative, perhaps these collagen-like sequences serve as ligands for the α2β1 integrin when these proteins are bound to microbial surfaces. Experiments are under way to further study in vitro PMC α2β1 integrin–dependent responses.
Previous studies have addressed the roles of the α2β1 integrin during different models of inflammation.4-6 In vivo treatment of mice with inhibitory anti-α2β1 integrin monoclonal antibody was shown to inhibit a delayed-type hypersensitivity reaction, a contact hypersensitivity reaction (a specific version of a delayed type hypersensitivity reaction), and a model of collagen-induced arthritis.4 These authors suggested, but could not explicitly prove, that the mechanism of action of the anti-α2β1 integrin antibody was at the level of the infiltrating, activated T lymphocyte. In light of our data demonstrating a role for α2β1 integrin on mast cells in vivo, it is also possible that anti-α2β1 integrin antibody was inhibiting mast cell responses in this report. Both delayed-type hypersensitivity and arthritis models have been shown to require mast cells for full responses.46-49 Therefore, these experiments ascribing a function to α2β1 integrin on the infiltrating T lymphocyte require re-evaluation.
In conclusion, we demonstrate a requirement for α2β1 integrin during mast cell–mediated inflammatory responses to infection in vivo. Whether the α2β1 integrin serves a similar role during other mast cell–mediated pathogenic processes, such as autoimmune arthritis or asthma, is currently being explored. If so, α2β1 integrin could serve as a therapeutic target for treatment of these diseases.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-08-2978.
Supported by NIH grants RO1, CA70275, and CA98027.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Bruce Linders and Javier Carrero for expert technical assistance and Laura Wells for mouse breeding. We thank Drs Emil R. Unanue and Samuel A. Santoro for advice in many helpful discussions.

![Figure 5. Mast cell transfer experiments confirm a requirement for α2β1 integrin on PMCs during the inflammatory response to Listeria. (A,B) Representative flow cytometric profiles of uninfected mast cell–deficient WBB6F1-W/Wv mice reconstituted with wild-type (A) or α2 integrin–deficient (B) PMCs. Reconstituted, uninfected mice contained equivalent numbers of total peritoneal cells (5.6 × 106 [0.21%] for WT reconstituted mice and 5.2 × 106 [0.26%] for KO reconstituted mice) and equivalent percentages of PMCs (approximately 0.2%). (C,D) Mast cell–deficient mice reconstituted with either wild-type (WT) or α2 integrin–deficient (KO) mast cells were infected with 5 × 104 Listeria intraperitoneally. At 6 hours after infection, the percentage of PMN and the absolute PMN number were determined. The average total peritoneal cell number in reconstituted, infected mice was as follows: 8.1 × 106 in WT reconstituted mice and 5.6 × 106 in KO reconstituted mice. Shown is a combination of 2 experiments. Uninfected groups each contained 2 mice. Infected groups contained 4-5 mice. Results are presented as means ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2003-08-2978/6/m_zh80060458580005.jpeg?Expires=1767809081&Signature=WlVC7Dcn7iAhJbHFR~mGVSmKwxYhZ4da5TpswkLxO667yk-X3lUGCbOPoHOc~Zixbq1ZxHIgIa230HTdsEBVOsTeXf4BZLOPm~wBZTkF1eG1NoDQ~bUa7~uxHDI2zB2Oh17Nb1WNIkizOqe-tcmv8gxB0eVPuHbVz7SXUxRQrY-FygHz4M-VvIVVGVH1Oh-7tGbTzTWy8XU7dMvSDmkmH2iSxTW4v8MFBFsvQfbee8dYJ~bycJy-RdnW9IUkm75jx-rmgv53ialcudHTjAb4TgroP5ZLQOnJXRWZli8H1fFOaD9CRSn9bt~v-fpgBFcR50vp~xZgnSoT1ySDi35ejg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)