Abstract
Interferon consensus sequence-binding protein (ICSBP) is a transcription factor belonging to the interferon regulatory factor (IRF) family, recently shown to play a critical role in dendritic cell (DC) differentiation. Here, we analyzed the role of ICSBP in the development and trafficking of epidermal Langerhans cells (LCs) and dermal DCs and the implications for initiation of a competent immune response. ICSBP-/- mice exhibited a reduced frequency of LCs and a delayed mobility of DCs from skin that reflected a slower turnover rate in lymph nodes during steady-state conditions. Even under inflammatory changes, ICSBP-/- DCs displayed reduced mobility from skin to lymph nodes and, as a consequence, failed to induce a contact hypersensitivity (CHS) response, suggesting that these DCs were unable to initiate a competent antigen (Ag)–specific T-cell–mediated immunity. Moreover, bone marrow (BM)–derived DCs from ICSBP-/- mice exhibited an immature phenotype and a severe reduction of interleukin 12 (IL-12) expression. These BM DCs also showed a marked defect in their migratory response to macrophage inflammatory protein 3α (MIP-3α), MIP-3β, and the CC chemokine CCL21/6Ckine, which was paralleled by an impaired expression of the CC chemokine receptors, CCR6 and CCR7. Together, these results indicate that ICSBP is critically required for the development and trafficking of skin DCs, thus playing a critical role in the DC-mediated initiation of T-cell immunity.
Introduction
Dendritic cells (DCs) represent a heterogeneous network of professional antigen-presenting cells (APCs) that function as sentinels of the immune system.1 The different bone marrow (BM)–derived subtypes of DCs traffic as precursors through the blood stream to tissues, where they become resident immature DCs such as epidermal Langerhans cells (LCs) and dermal DCs in the skin. At these sites, they capture antigens and become activated following inflammatory stimuli. As a consequence, skin DCs migrate to draining lymphoid organs and home to the T-cell–rich areas, where they are endowed with potent stimulatory capacity to prime naive T cells.2 During migration, all DC subtypes undergo major changes in phenotype and function. Immature DCs express a number of CC chemokine receptors (CCR1, CCR2, CCR5, and CCR6) for inducible chemokines, such as macrophage inflammatory protein 3α (MIP-3α) and RANTES (regulated on activation, normal T expressed, and secreted), by which they are attracted to sites of inflammation.3-7 Importantly, each immature DC population displays a unique spectrum of chemokine responsiveness, as LCs migrate selectively to MIP-3α, which is the only chemokine detected within inflamed epithelium.8 Upon maturation, DCs lose their responsiveness to most of the inflammatory chemokines through receptor down-regulation but up-regulate other receptors, such as CCR4 and CCR7, for constitutively expressed chemokines that allow their homing to lymph nodes.9 In this regard, it has been shown that the constitutive production of the CC chemokine CCL21/6Ckine by lymphatic endothelium provides the first chemotactic gradient for antigen-loaded CCR7-positive DCs, leading to their selective recruitment from the site of infection into the lymph stream.10 Once activated, DCs enter the lymphatics and a chemotactic gradient of 6Ckine and CCL19/MIP-3β, which are produced by DCs and stromal cells in the T-cell zone, drives their localization to the paracortical area of the lymph nodes.11 Recently, many studies have highlighted the important role of the chemokine system in DC migration during steady-state conditions and inflammation.12 However, the events linking the development of DC populations and their trafficking properties are not yet fully elucidated.
Interferon consensus sequence-binding protein (ICSBP), also known as interferon regulatory factor 8 (IRF-8), is a transcription factor belonging to the IRF family that plays a critical role in the regulation of lineage commitment, especially in myeloid cell differentiation.13 It is expressed in BM progenitor cells and controls the cell growth and differentiation of myeloid cells at different developmental stages.13-15 It has been reported that ICSBP can affect the proliferative potential of myeloid cells at the progenitor cell level, playing a role in promoting macrophage differentiation while inhibiting the development of granulocytes.16,17 In addition, myeloid cells from ICSBP-/- mice have been reported to exhibit defective apoptosis.18 We recently reported that ICSBP acts as a key factor in controlling in vivo the developmental maturation program of plasmacytoid DCs, also called interferon-producing cells (IPCs).19 This finding is in agreement with in vitro studies showing that ICSBP-/- BM progenitor cells were defective in generating IPCs in the Fms-like tyrosine kinase 3 ligand (Flt3-L)–based culture system.20 Moreover, recent studies have shown that ICSBP is important for the in vivo differentiation and activation of CD8α+ DCs and may also influence the functional maturation of CD8α- subsets.19,21,22 Altogether, these data indicate that ICSBP is critically required for the development and the maturation of defined DC populations.
In the present study, we have investigated the role of ICSBP in the trafficking and activities of DC populations. In particular, we focused on the role of this transcriptional factor in the development and migration of LCs and dermal DCs, which represent the highly specialized DCs localized in the skin. We found that ICSBP-/- DCs exhibit a defective mobilization from skin in steady-state conditions and during inflammation, leading to a defective contact hypersensitivity (CHS) reaction. Furthermore, BM-derived DCs from ICSBP-/- mice show immature phenotype, as well as defective interleukin 12 (IL-12) expression, and an impaired migratory response to chemokines, which may affect the homing of DCs in the skin. Overall, we provide the first evidence that ICSBP plays a critical role in regulating DC migration and, subsequently, the initiation of acquired antigen (Ag)–specific T-cell–mediated immunity.
Materials and methods
Mice
ICSBP-deficient mice were generated as described.23 Homozygous deficient (-/-) and wild-type (WT) mice (+/+) on a (C57BL/6 × 129/Sv) F2 background were bred and maintained under specific pathogen-free conditions. All mice were treated according to the guidelines for animal treatment at central animal facilities at the Instituto Superiore di Sanita (ISS; Rome, Italy) and the laboratory holds permission to perform animal experiments described.
Dendritic cell isolation from lymphoid organs
DCs were generated from bone marrow cells as described previously.24 Briefly, bone marrow was extracted from tibiae and femurs and cell suspensions were cultured in Iscove modified Dulbecco medium (IMDM) medium (Cambrex, East Rutherford, NJ) containing 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF; Peprotech, London, United Kingdom). Fresh medium and cytokine were given every 2 to 3 days. On days 3 to 7, loosely adherent cells were harvested and used for experiments. DC isolation from cutaneous lymph nodes was performed as previously described.25 In brief, cutaneous (mandibular, axillary, and inguinal) lymph nodes from 3 to 5 mice were cut into small fragments and digested in RPMI 1640 (Cambrex) containing 10% fetal calf serum, 1 mg/mL type III collagenase (Worthington Biochemical Corporation, Lakewood, NJ), and 325 kilo units per milliliter DNase I (Sigma, St Louis, MO), with periodic pipetting to break up fragments, for 25 minutes at room temperature. EDTA (ethylenediaminetetraacetic acid; 0.1 M, pH 7.2; Sigma) was added for an additional 5 minutes to allow disruption of DC–T-cell complexes. Cells were washed, resuspended in Nycodenz (1.077 g/mL; Life Technologies, Paisley, United Kingdom), overlaid on an additional layer of Nycodenz, and centrifuged at 1700g for 20 minutes. The low-density fraction was collected, washed, and used for phenotypic analysis.
mAbs and flow cytometry
The following monoclonal antibodies (mAbs; all from BD Pharmingen, San Diego, CA) were used: anti-CD40–biotin (HM40-3), anti-CD80–biotin (16-10A1), anti-CD86–biotin (GL1), anti–I-Ad/I-Ed–biotin (2G9), and anti-CD11c (HL3), which was used in either phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–conjugated form. Biotinylated mAbs were detected with streptavidin–peridinin chlorophyll A protein (PerCP) (BD Pharmingen), streptavidin-Red670 (Life Technologies), or streptavidin-Tricolor (Caltag Laboratories, Burlingame, CA). Stained cells were analyzed on a FACSort flow cytometer (Becton Dickinson, San Jose, CA). Viable cells were selected for analysis based on forward- and side-scatter properties.
In vitro migration assay
In vitro migration in response to chemokines was evaluated using a chemotaxis chamber technique. Bone marrow–derived DCs were washed and plated on the upper compartment of 5-μm pore size Transwell plates (Costar, High Wycombe, United Kingdom) at 4 × 105 cells per well in 100 μL serum-free IMDM. One hundred nanograms per milliliter of RANTES, MIP-3β, 6Ckine (all from R&D Systems, Minneapolis, MN), MIP-3α (Peprotech), or control serum-free IMDM were added to the lower chamber. After 3 hours of incubation at 37°C, cells that had transmigrated to the bottom compartment were collected and enumerated in Neubauer counting chamber. Each experiment was performed in triplicate. In some experiments, transmigrated cells were stained with anti–I-A/I-E and anti-CD11c antibodies and analyzed by fluorescence-activated cell sorter (FACS). Calculation of absolute numbers of double-positive cells was performed by applying the percentage of cells coexpressing the CD11c and I-A markers to the mean number of transmigrated cells of the triplicate Transwell culture plates.
LC isolation and ex vivo migration of dendritic cells from ear skin explants
The procedure was adapted from that described by other authors.26,27 Ears were removed from groups of at least 3 mice and split into dorsal and ventral halves. Ventral halves were rinsed with 90% ethanol and then placed separately in 1 mL 0.25% trypsin (Invitrogen Life Technologies, Carlsbad, CA) for 1 hour at 37°C. After extensive washing in phosphate-buffered saline (PBS), epidermis was separated from dermis with the help of a scalpel. For LC isolation, the trypsinized epidermal sheets were filtered through a stainless-steel sieve. Cell suspensions were collected, passed through a 70-μm cell strainer (BD Falcon, Heidelberg, Germany), and centrifuged on a Nycodenz gradient. The LC-enriched low-density fraction was then collected and used for phenotypic analysis. For ex vivo emigration assay, both dermal and epidermal sheets were singularly placed split-side down in 24-well culture plates with 1 mL 10% FCS-IMDM medium alone, or medium containing rmGM-CSF (100 ng/mL) or 6Ckine (100 ng/mL), and incubated at 37°C. Twenty-four to 48 hours later, sheets were removed and cells that migrated into the culture medium were harvested, counted, and subsequently FACS stained with anti-CD11c coupled with anti–I-A/I-E, anti-CD86, or anti-CD40 alternatively. The absolute numbers of double-positive cells were calculated as described above.
In vivo skin sensitization with FITC
Groups of 3 to 5 mice were painted on the abdomen with 200 μL 1% FITC (Sigma) dissolved in a solvent of acetone-dibutylphtalate, 1:1. In some experiments, 10 μL of the same FITC solution was applied on the dorsal side of each ear. Twenty-four to 48 hours after FITC painting, the skin-draining lymph nodes (inguinal or auricular, depending on the site of FITC application) were removed and enriched for DCs, as described in “In vitro migration assay.” Cells were then double-stained with anti-CD11c and anti-CD40 or anti–I-A/I-E and analyzed by FACS to detect FITC-bearing DCs in lymph nodes (LNs). Ears from mice receiving FITC 24 hours before were the source of epidermal sheets used to assess the emigration of LCs after skin sensitization.
Elicitation of a contact hypersensitivity (CHS) response
The approach was similar to that described by others.28,29 In brief, 200 μL of 1% FITC solution was applied on the abdomens of at least 4 mice. Six days later, mice were challenged by painting the dorsal side of one ear with 10 μL of FITC. CHS response was determined 24 hours after challenge by measuring ear thickness using a dial thickness micrometer. Ear swelling was calculated by subtracting the thickness of the ear that was not subjected to FITC challenge. To determine the specific swelling, ear thickness was also measured in a group of mice that were not sensitized but were challenged.
BrdU labeling and analysis of DC turnover in cutaneous LNs
The procedure was similar to that described by Kamath et al.30 Mice were injected intraperitoneally with 1 mg bromodeoxyuridine (BrdU; Sigma) and were fed thereafter with 0.8 mg/mL BrdU dissolved in drinking water, which was changed daily. After various days, groups of 3 mice were killed and skin-draining LNs were removed. DCs were enriched and surface stained, as described above. Cells were then fixed in cold 95% ethanol for 30 minutes, washed, and then incubated for 30 minutes at room temperature in 1% paraformaldehyde, 0.01% Tween 20 in PBS. DCs were then pelleted and incubated for 10 minutes at room temperature with 50 U DNase I (Sigma) in a 0.15 M NaCl, 4.2 mM MgCl2 solution. After washing, cells were stained with FITC-conjugated anti-BrdU mAb (Becton Dickinson), washed, and analyzed by FACS.
RT-PCR and analysis of amplified products
Total RNA was extracted from 2 × 106 to 5 × 106 BM DCs by using the miniprep total RNA purification kit (Qiagen, Hilden, Germany). RNA (500 ng) was incubated at 25°C for 10 minutes with Oligo-p(dT)15 (Roche Diagnostics, Mannheim, Germany) in the presence of 50 U RNase inhibitors (Roche Diagnostics) and reverse-transcribed using 20 U avian myeloblastosis virus (AMV) reverse transcriptase (Roche Diagnostics) for 1 hour at 42°C in a final volume of 20 μL (10 mM Tris, 50 mM KCl, 5 mM MgCl2, 1 mM deoxynucleoside triphosphates [dNTPs]; pH 8.3). Polymerase chain reaction (PCR) was performed on 2 μL of each cDNA sample, or as controls either 2 μL sterile water or 2 μL total RNA that had not been reverse transcribed, by means of 1.25 U Thermoprime Plus DNA polymerase (Advanced Biotechnologies, Epsom, United Kingdom) in a final volume of 50 μL containing 75 mM Tris, 20 mM ammonium persulphate, 0.1% Tween 20, 1.5 mM MgCl2, 0.2 mM dNTPs, 10 pmol sense primer, and 10 pmol antisense primer, pH 8.8. The specific primer pairs used were as follows. IL-12 (p40 subunit): 5′-AACTGGCGTTGGAAGCACGG-3′ (sense) and 5′-GAACACATGCCCACTTGCTG-3′ (antisense); CCR1: 5′-TTTTAAGGCCCAGTGGGAGTTCACTCACCG-3′ (sense) and 5′-TGGTATAGCCACATGCCTTTGAAACAGCTGC-3′ (antisense); CCR5: 5′-TACCAGATCTCAGAAAGAAGGTTTTCATTA-3′ (sense) and 5′-GCGTTTGACCATGTGTTTTCGGAAGAACACT-3′ (antisense); CCR6: 5′-ACTCTTTGTCCTCACCCTACCG-3′ (sense) and 5′-ATCCTGCAGCTCGTATTTCTTG-3′ (antisense); CCR7: 5′-ACAGCGGCCTCCAGAAGAACAGCGG-3′ (sense) and 5′-TGACGTCATAGGCAATGTTGAGCTG-3′ (antisense); β-actin: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ (sense) and 5′-CTAGAAGCATTGCGGTGGAGCATGGAGGG-3′ (antisense). All primers were obtained from Invitrogen Life Technologies. The samples were amplified for 20 to 30 cycles at different annealing temperatures, optimal to each primer combination. Amplified products (10 μL) were separated by agarose gel electrophoresis on a 1% Tris-acetate-EDTA (TAE) gel and visualized by ethidium bromide staining and UV transillumination. β-Actin reverse transcriptase–PCR (RT-PCR) was run in parallel to normalize the levels of mRNA in the samples. The signal intensity of amplified bands was determined by LKB XL Ultroscan densitometer (Pharmacia, Uppsala, Sweden).
Statistical analysis
Data were expressed as the mean ± SE. Statistical analyses were performed using 2-tailed Student t test.
Results
Impaired skin DC trafficking in ICSBP-/- mice in steady state
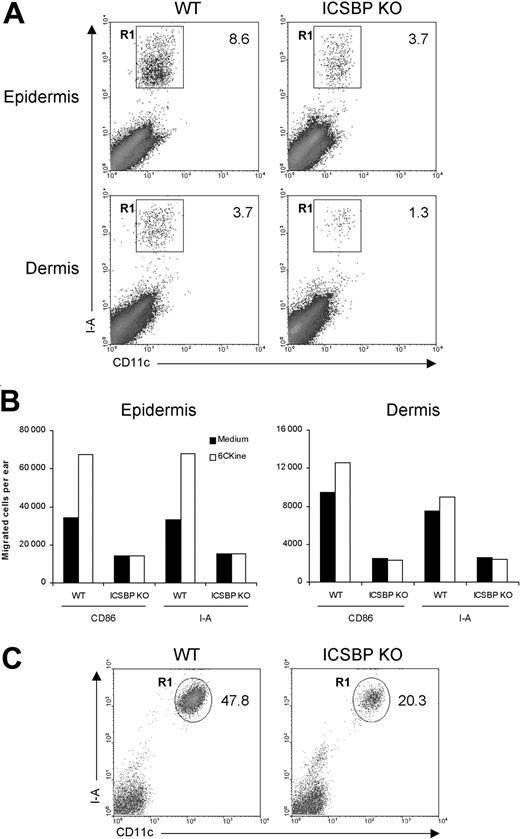
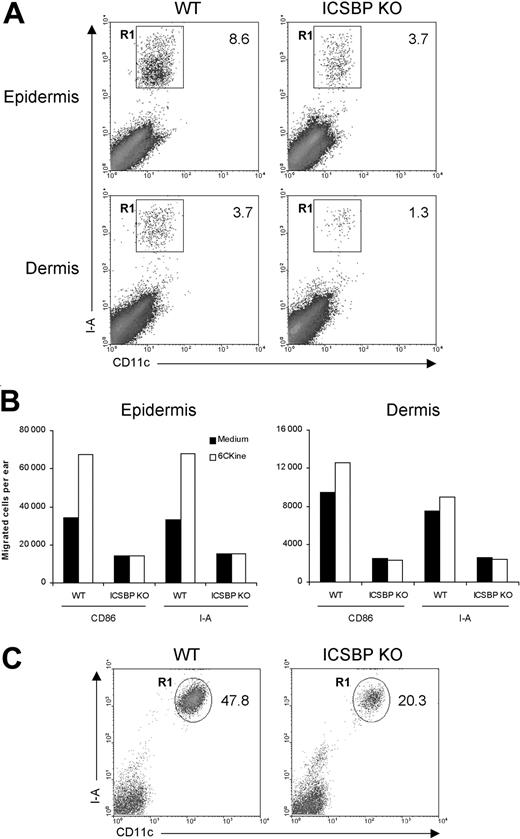
First, we examined the ability of skin DCs from ICSBP-/- mice to respond to migratory signals under steady-state conditions. To this end, we used skin explants, which are known to be rich in DCs.31 Epidermal and dermal sheets isolated from the ears of ICSBP-/- and WT mice were singularly cultured for 24 hours in the presence of GM-CSF to elicit DC migration from tissues. As DCs represent less than 5% of skin components, we subsequently stained emigrated cells with DC-specific markers. As illustrated in Figure 1A, the percentage of DCs, identified as CD11c+/I-A+, migrating in response to GM-CSF was markedly reduced in skin explants from ICSBP-/- compared with WT mice, with 2.3-fold and 2.8-fold differences for epidermis and dermis, respectively. To evaluate the migratory potential of ICSBP-/- skin DCs in response to a chemotactic signal, we incubated skin explants for 48 hours in medium alone or in presence of 6Ckine. In this case, we used 2 different markers (I-A or CD86) alternatively coupled with CD11c to detect DCs. Expectedly, addition of 6Ckine to WT tissues resulted in increased migration of DCs compared with tissues cultured in medium alone (Figure 1B). This was particularly evident in the epidermis, while dermal DCs showed a slight chemotactic response. In contrast, no significant specific response to 6Ckine was found in either epidermis or dermis from ICSBP-/- mice. Moreover, the number of migratory DCs found in ICSBP-/- skin explants cultured in medium alone was significantly lower with respect to the WT counterparts (Figure 1B). On the basis of these data, we asked whether the impaired migration of ICSBP-/- DCs from skin explants could also reflect a reduced number of DCs in the skin of these mice. To test this possibility, we analyzed the presence of LCs in freshly isolated epidermis. As revealed by CD11c and I-A expression, the number of LCs was significantly lower in the epidermis of ICSBP-/- compared with WT mice (Figure 1C).
Frequency and migration of DCs from ear skin in ICSBP-/- mice. Ears from 3 to 5 ICSBP-/- and WT mice were excised and split into dorsal and ventral halves. Dorsal halves were further treated, as indicated in “Materials and methods,” to allow the separation of dermis from epidermis. (A) Dermal and epidermal sheets were incubated ventral-side down in medium containing GM-CSF for 24 hours, then cells that migrated from the tissue into the culture medium were collected and double-stained for CD11c and I-A expression. R1 region indicates the percentage of double-positive cells within the total population of living cells; KO, knock-out. (B) Dermis and epidermis were incubated in presence or absence of 6Ckine. Forty-eight hours later, cells that migrated ex vivo were collected, counted, and stained for CD11c coupled with the indicated antibodies. The number of positive cells was calculated by applying the percentage of double positivity to the mean number of migrated cells. (C) Freshly isolated epidermal sheets were passed through a steel sieve and cell suspensions were centrifuged on a Nycodenz gradient. The low-density fraction was collected and stained for identification of LCs. Representative data of 1 of 3 experiments are shown.
Frequency and migration of DCs from ear skin in ICSBP-/- mice. Ears from 3 to 5 ICSBP-/- and WT mice were excised and split into dorsal and ventral halves. Dorsal halves were further treated, as indicated in “Materials and methods,” to allow the separation of dermis from epidermis. (A) Dermal and epidermal sheets were incubated ventral-side down in medium containing GM-CSF for 24 hours, then cells that migrated from the tissue into the culture medium were collected and double-stained for CD11c and I-A expression. R1 region indicates the percentage of double-positive cells within the total population of living cells; KO, knock-out. (B) Dermis and epidermis were incubated in presence or absence of 6Ckine. Forty-eight hours later, cells that migrated ex vivo were collected, counted, and stained for CD11c coupled with the indicated antibodies. The number of positive cells was calculated by applying the percentage of double positivity to the mean number of migrated cells. (C) Freshly isolated epidermal sheets were passed through a steel sieve and cell suspensions were centrifuged on a Nycodenz gradient. The low-density fraction was collected and stained for identification of LCs. Representative data of 1 of 3 experiments are shown.
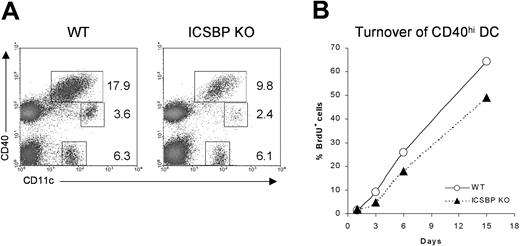
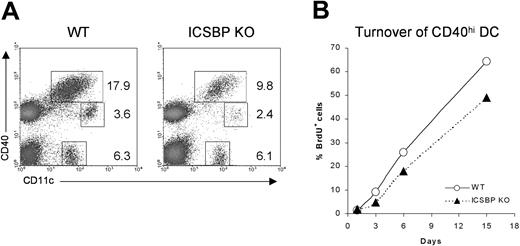
Although the trafficking of skin DCs under steady-state conditions still remains poorly understood, it has been shown that epidermal- and dermal-derived DCs display high expression of CD40 and I-A molecules when they reach the draining LNs.32,33 To test whether the impaired number and migration observed in skin cultures from ICSBP-/- mice could reflect a defect in homing of DCs from skin to LNs, we studied the distribution of the DC populations in cutaneous LNs from ICSBP-/- mice (Figure 2A). We distinguished 3 different DC subsets: CD11c+CD40hi, CD11c+CD40lo, and CD11c+CD40-. Of interest, the CD11c+ CD40hi subset, which includes the skin-derived DCs, showed a 50% reduction in LNs of ICSBP-/- compared with WT mice.
Analysis of skin-derived DC populations in cutaneous LNs from ICSBP-/- mice. (A) Skin-draining lymph nodes from ICSBP-/- and WT mice were enriched on Nycodenz and double-stained for CD11c and CD40 expression. Dot plots represent DC populations identified by CD11c and CD40 expression. Values are calculated on the population of LN suspensions from at least 5 mice. (B) ICSBP-/- and WT animals were given BrdU in drinking water for 15 days. At the indicated time points, cutaneous LNs were removed and triple-stained for determination of BrdU uptake in the CD40hi CD11c+ DC subset. Data are representative of 2 separate experiments.
Analysis of skin-derived DC populations in cutaneous LNs from ICSBP-/- mice. (A) Skin-draining lymph nodes from ICSBP-/- and WT mice were enriched on Nycodenz and double-stained for CD11c and CD40 expression. Dot plots represent DC populations identified by CD11c and CD40 expression. Values are calculated on the population of LN suspensions from at least 5 mice. (B) ICSBP-/- and WT animals were given BrdU in drinking water for 15 days. At the indicated time points, cutaneous LNs were removed and triple-stained for determination of BrdU uptake in the CD40hi CD11c+ DC subset. Data are representative of 2 separate experiments.
To better understand the homing of skin DCs in vivo, we studied how quickly the CD11c+CD40hi skin-derived DCs turned over in cutaneous LNs of ICSBP-/- mice by monitoring BrdU incorporation (Figure 2B). It has been described that skin-derived CD11c+CD40hi cells recycle completely within 1 month, much slower than other DC subsets in LNs, which turn over within 10 days.32 We too found that CD11c+CD40hi turned over slower than other DC subsets in WT mice (data not shown). Indeed, approximately 67% of CD11c+CD40hi from lymph nodes of WT animals were BrdU+ after 15 days. In contrast, only 50% of the same cells from ICSBP-/- mice had turned over by the same time (Figure 2B), suggesting an impaired trafficking of skin DCs in vivo.
Defective migration of skin DCs from ICSBP-/- mice during inflammation
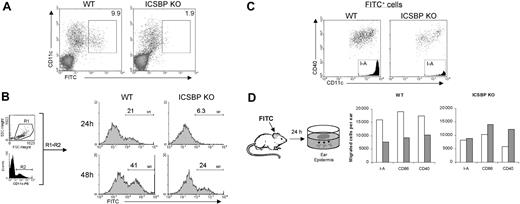
We next investigated the ability of skin DCs from ICSBP-/- mice to migrate during inflammation. Cutaneous sensitization with hapten compounds, like FITC dissolved in an irritant solution, is known to resemble an inflammatory stimulus and to induce the migration of both dermal and epidermal DCs into the draining lymph nodes where they can be detected as early as 24 hours after application of FITC.34 Thus, FITC was applied to the abdomen of WT and ICSBP-/- mice, and 24 hours later the presence of FITC-bearing CD11c+ cells in the inguinal LNs was analyzed by FACS. As shown in Figure 3A, FITC-bearing DCs were barely detectable in ICSBP-/- animals, whereas a consistent number of CD11c+FITC+ cells could be detected in LNs from WT mice. Interestingly, the presence of CD11c+FITC+ cells in LNs from ICSBP-/- mice was detected with a delay of 24 hours compared with the WT counterparts (Figure 3B). In fact, 21% of total CD11c+ cells were FITC+ in LNs from WT mice 24 hours after sensitization, which approximately doubled after 48 hours. Conversely, few FITC-bearing CD11c+ cells were present in LNs from ICSBP-/- animals at 24 hours, but by 48 hours 24% were positive, approximately the same frequency observed in WT animals at 24 hours (Figure 3B). Of note, the phenotype of FITC-bearing cells found in LNs of sensitized mice, both WT and ICSBP-/-, was that typical of skin-derived DCs32,33 as shown by high expression of CD40 as well as I-A (Figure 3C).
Defective DC mobilization from skin to draining LNs in ICSBP-/- mice upon FITC sensitization in vivo. (A-B) Mice were sensitized with FITC on the shaved abdomen. Twenty-four hours later (A), draining inguinal LNs were removed and cell suspensions were subjected to Nycodenz centrifugation to enrich for DCs. Cells were then stained with PE-labeled anti-CD11c antibody and analyzed by FACS for the presence of DCs that had migrated from the skin (FITC+). Density plots were gated to exclude dead cells. (B) Draining LNs were removed 24 hours or 48 hours after skin sensitization with FITC. Histograms represent the expression of FITC (M1) on cells gated on forward-side scatter properties (R1) and on CDIIc positivity (R2). (C) Draining lymph nodes from 48-hour FITC-treated mice were enriched on Nycodenz and double-stained with anti-CD11c coupled with anti-CD40 or anti–I-A, alternatively. Dot plots and histograms show the population gated on the basis of FITC positivity. (D) FITC was applied to ears of ICSBP-/- and WT mice. After 24 hours, epidermal sheets were incubated for an additional 24 hours in medium containing GM-CSF to allow the release of epidermal cells from the tissue. Cells emigrated in the culture medium were collected, counted, and subjected to FACS analysis. Histograms represent the number of cells expressing the indicated markers in cultured epidermal sheets of untreated (□) or FITC-treated (▦) mice. Representative data of 1 of 3 separate experiments are shown.
Defective DC mobilization from skin to draining LNs in ICSBP-/- mice upon FITC sensitization in vivo. (A-B) Mice were sensitized with FITC on the shaved abdomen. Twenty-four hours later (A), draining inguinal LNs were removed and cell suspensions were subjected to Nycodenz centrifugation to enrich for DCs. Cells were then stained with PE-labeled anti-CD11c antibody and analyzed by FACS for the presence of DCs that had migrated from the skin (FITC+). Density plots were gated to exclude dead cells. (B) Draining LNs were removed 24 hours or 48 hours after skin sensitization with FITC. Histograms represent the expression of FITC (M1) on cells gated on forward-side scatter properties (R1) and on CDIIc positivity (R2). (C) Draining lymph nodes from 48-hour FITC-treated mice were enriched on Nycodenz and double-stained with anti-CD11c coupled with anti-CD40 or anti–I-A, alternatively. Dot plots and histograms show the population gated on the basis of FITC positivity. (D) FITC was applied to ears of ICSBP-/- and WT mice. After 24 hours, epidermal sheets were incubated for an additional 24 hours in medium containing GM-CSF to allow the release of epidermal cells from the tissue. Cells emigrated in the culture medium were collected, counted, and subjected to FACS analysis. Histograms represent the number of cells expressing the indicated markers in cultured epidermal sheets of untreated (□) or FITC-treated (▦) mice. Representative data of 1 of 3 separate experiments are shown.
To test if the observed delay of DC migration from skin to LNs in ICSBP-/- mice reflected a slow mobilization from skin in response to FITC, we analyzed the frequency of dendritic cells in the skin after FITC sensitization in vivo. To this end, mice were sensitized with FITC on the dorsal side of each ear. Twenty-four hours later, ears were excised and epidermal sheets were cultured in presence of GM-CSF to allow the release of cells from the tissue. After 24 hours of culture, emigrated cells were collected, counted, and stained to identify DCs. Interestingly, the number of DCs in ear epidermis of FITC-treated WT animals were less than 50% of the levels of control unsensitized animals, as revealed by the number of I-A+, CD40+, and CD86+ cells (Figure 3D). These data suggest that a portion of DCs had already moved from the epidermis due to FITC stimulation and are consistent with the observed accumulation of FITC+ DCs in the draining LNs of WT mice 24 hours after skin sensitization (Figure 3A). In contrast, no drop in DC number was observed in epidermal sheets from ICSBP-/- mice treated with FITC with respect to unsensitized controls, implying an absence of mobilization of DCs from skin to LNs following the inflammatory stimulus (Figure 3A,D).
Lack of a CHS response in ICSBP-/- mice
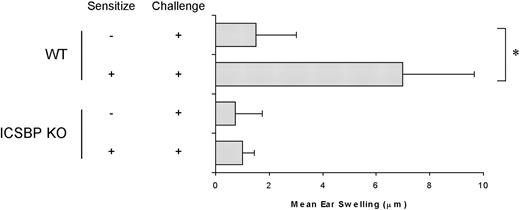
To investigate whether the defect in skin DC migration resulted in an altered induction of DC-dependent immune responses in ICSBP-/- mice, we measured the CHS response to FITC sensitization (Figure 4). Strikingly, CHS response was completely inhibited in ICSBP-/- mice, as revealed by the absence of net ear swelling after FITC challenge. In contrast, WT animals displayed a 4.5-fold increase in ear thickness in response to FITC challenge, compared with nonsensitized controls, indicative of a CHS response.
Defective CHS response in ICSBP-/- mice. Mice were sensitized to FITC on the abdomens and challenged on one ear 6 days later. Ear thickness was measured using an engineers' micrometer 24 hours after challenge on both ears. Ear swelling was calculated by subtracting the thickness of the unchallenged ear from that of the FITC-challenged ear in each individual animal. Control swelling was obtained from mice subjected to challenge but not sensitized. Results are expressed as the mean of at least 5 mice per group ± SE (*P < .01).
Defective CHS response in ICSBP-/- mice. Mice were sensitized to FITC on the abdomens and challenged on one ear 6 days later. Ear thickness was measured using an engineers' micrometer 24 hours after challenge on both ears. Ear swelling was calculated by subtracting the thickness of the unchallenged ear from that of the FITC-challenged ear in each individual animal. Control swelling was obtained from mice subjected to challenge but not sensitized. Results are expressed as the mean of at least 5 mice per group ± SE (*P < .01).
BM DCs from ICSBP-/- mice display altered chemotactic response
We next asked whether the defective migratory behavior of skin DCs in ICSBP-/- mice could reflect an altered developmental pathway of BM progenitors. To test this, we examined the differentiation of DCs from BM cultures in the presence of GM-CSF. This in vitro system allows for the differentiation of myeloid DCs while impeding the development of plasmacytoid and the so-called lymphoid DCs.35,36 After 6 days of culture, cells expressing the CD11c marker indicative of DCs can comprise 60% to 70% of the total population (Montoya et al24 and data not shown). Even though cultures from WT and ICSBP-/- mice displayed similar numbers of CD11c+ cells after 6 or 7 days of culture (not shown), the phenotype of the ICSBP-/- DCs was markedly altered. In fact, the BM-derived ICSBP-/- DCs did not express CD40 and displayed significantly lower levels of CD80, CD86, and major histocompatibility complex (MHC) class II molecules when compared with WT DCs (Figure 5A). To further characterize the maturation of BM DCs in ICSBP-/- mice, we analyzed the expression of IL-12, a cytokine known to be up-regulated during BM DC maturation.37 To this end, RNA was extracted from bone marrow at the beginning (day 0) and after 3 or 7 days of culture in presence of GM-CSF and assayed for IL-12p40 expression by RT-PCR. As expected, WT DCs exhibited increasing levels of IL-12p40 transcripts during maturation with very high expression levels at day 7. In contrast, IL-12p40 was barely expressed even after 7 days of culturing BM DCs from ICSBP-/- mice (Figure 5B), indicating a defective maturation of DCs from the bone marrow in these animals.
Phenotype of BM-derived DCs from ICSBP-/- mice. BM from ICSBP KO and control WT animals was cultured for 7 days in presence of GM-CSF. (A) BM DCs were double-stained for CD11c and a panel of antibodies specific for the indicated markers. Data represent the expression of the indicated markers in the population gated on forward-side scatter properties and on CD11c positivity. Gray-filled graphs represent the specific staining for the indicated markers, while broken lines are the isotype-matched controls. (B) RT-PCR analysis of IL-12p40 expression at various times of BM culture. Day 0 represents the time of bone marrow extraction. Representative data of 1 of 3 separate experiments are shown.
Phenotype of BM-derived DCs from ICSBP-/- mice. BM from ICSBP KO and control WT animals was cultured for 7 days in presence of GM-CSF. (A) BM DCs were double-stained for CD11c and a panel of antibodies specific for the indicated markers. Data represent the expression of the indicated markers in the population gated on forward-side scatter properties and on CD11c positivity. Gray-filled graphs represent the specific staining for the indicated markers, while broken lines are the isotype-matched controls. (B) RT-PCR analysis of IL-12p40 expression at various times of BM culture. Day 0 represents the time of bone marrow extraction. Representative data of 1 of 3 separate experiments are shown.
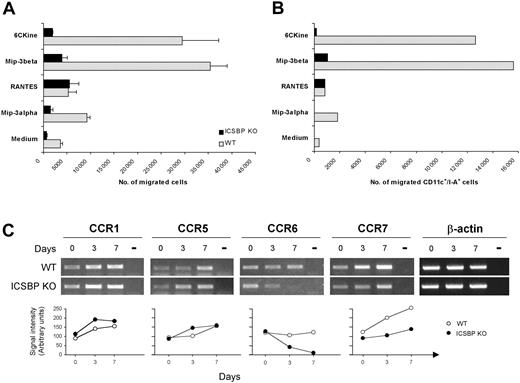
To determine whether the impaired phenotype observed in BM DCs from ICSBP-/- mice could influence their ability to respond to migratory stimuli, we assayed the chemotactic response of 7-day cultures to a range of chemokines known to act on DCs at different developmental stages. As shown in Figure 6A, WT DCs displayed marked chemotactic responses to 6Ckine and MIP-3β, exhibiting 8- to 10-fold increases, respectively, of migrated cells compared with cells incubated in medium alone. In contrast, BM-derived ICSBP-/- DCs showed significantly lower migratory response to both 6Ckine and MIP-3β, with 2.5-fold and 4-fold increase, respectively, compared with control cells. In addition, WT cultures showed detectable migration in response to MIP-3α (3-fold increase), whereas this chemokine did not elicit significant migration of ICSBP-/- DCs. Of interest, ICSBP-/- DCs retained normal chemotactic response (7.5-fold) to RANTES, comparable to that observed in WT cultures (7-fold; Figure 6A).
Altered migration potential and expression of chemokine receptors in BM DCs from ICSBP-/- mice. (A-B) Seven-day cultured BM DCs were tested for in vitro chemotactic response using Transwell chambers. DCs were loaded on the upper chamber, while the indicated chemokines were added to the bottom wells. After a 3-hour incubation, cells that had migrated to the lower compartment were collected and counted. Data in panel A represent the mean number of migrated cells of triplicate cultures ± SE. (B) Migrated cells from triplicate cultures were pooled, double-stained with anti-CD11c and anti–I-A antibodies, and analyzed by FACS. (C) Total RNA was extracted from cell suspensions at various times of BM culture and subjected to RT-PCR, using specific primers. Day 0 represents the time of BM extraction. Transcript levels for the indicated chemokine receptors are shown in the top panel. Bottom panels show the densitometric analysis of RT-PCR bands normalized to β-actin levels. Results are representative of 3 separate experiments.
Altered migration potential and expression of chemokine receptors in BM DCs from ICSBP-/- mice. (A-B) Seven-day cultured BM DCs were tested for in vitro chemotactic response using Transwell chambers. DCs were loaded on the upper chamber, while the indicated chemokines were added to the bottom wells. After a 3-hour incubation, cells that had migrated to the lower compartment were collected and counted. Data in panel A represent the mean number of migrated cells of triplicate cultures ± SE. (B) Migrated cells from triplicate cultures were pooled, double-stained with anti-CD11c and anti–I-A antibodies, and analyzed by FACS. (C) Total RNA was extracted from cell suspensions at various times of BM culture and subjected to RT-PCR, using specific primers. Day 0 represents the time of BM extraction. Transcript levels for the indicated chemokine receptors are shown in the top panel. Bottom panels show the densitometric analysis of RT-PCR bands normalized to β-actin levels. Results are representative of 3 separate experiments.
As 7-day BM cultures also contained 30% to 40% non-DC contaminants, which could be represented by cells not yet differentiated and cell types committed to other lineages, it was important to analyze the phenotype of the migrated cells. Therefore, the migrated cells from triplicate cultures were pooled and stained with anti-CD11c coupled with anti–I-A or anti-CD86 mAbs and analyzed by FACS. As shown in Figure 6B, a considerable number of cells responding to chemokines in both WT and ICSBP-/- cultures were CD11c+/I-A+. In addition, WT DCs responding to 6Ckine and MIP-3β expressed high levels of CD86, indicative of a mature phenotype (not shown). Of interest, this analysis also revealed that in ICSBP-/- cultures no CD11c+/I-A+ DCs migrated in response to MIP-3α or 6CKine, while some migration could be detected in response to MIP-3β. In contrast, CD11c+/I-A+ DCs from ICSBP-/- mice displayed normal if not higher chemotactic response to RANTES with respect to the WT counterparts.
Chemokines are known to bind specific receptors, whose expression is finely regulated during the various stages of DC development.7 Therefore, we asked if the defective in vitro response to chemokines observed with BM DCs generated from ICSBP-/- mice could be explained by altered expression of chemokine receptors. RT-PCR and densitometric analysis revealed that CCR7 was strongly up-regulated during maturation of BM DCs from WT animals, consistently with their powerful response to the ligands MIP-3β and 6CKine (Figure 6), whereas CCR7 transcripts were only slightly increased in ICSBP-/- BM-derived DCs. In addition, while WT DCs expressed constantly detectable levels of MIP-3α receptor CCR6, these were markedly reduced at day 3 and disappeared completely at day 7 in ICSBP-/- cultures (Figure 6C). As expected, CCR1 and CCR5, which both bind RANTES,38 were expressed at comparable levels in ICSBP-/- and WT cultures. Taken together, these data indicate that BM DCs from ICSBP-/- mice are characterized by an altered phenotype and a selective impairment in the modulation of chemokine receptors during in vitro maturation, which may result in a defective response to migratory signals.
Discussion
It has been recently reported that ICSBP is critically required for the differentiation of plasmacytoid and of CD8α+ DCs and may also influence the functional maturation of CD8α- DCs.19-22 Here, we demonstrate that ICSBP plays a critical role in the development and maturation of skin DCs affecting their trafficking under steady-state conditions and during inflammation. Moreover, as a possibly linked process, we report that BM-derived DCs from ICSBP-/- mice display an immature phenotype along with a defective response to chemokines.
Skin DCs are BM-derived APCs and represent the first line of defense against environmental insults.9,39 Upon the receipt of danger signals, the capacity to respond to specific chemokines and subsequent migration to lymphoid tissues are fundamental features of skin DCs required for the initiation of an efficient immune response. Thus, the recruitment of epidermal and dermal DC precursors from the circulation into the skin, as well as the mobilization of skin DCs to the regional lymph nodes are tightly regulated events in vivo.3 In the present study, we report that ICSBP-/- mice exhibit an impaired trafficking of skin DCs, both in steady-state conditions and under inflammatory changes, with important consequences in the induction of T-cell–mediated immunity. The defective migration of skin ICSBP-/- DCs in steady state was initially unveiled by the significant decrease of DC emigration from both epidermis and dermis in GM-CSF– or 6Ckine-containing cultures. Moreover, we found that the number of LCs was significantly lower in the epidermis of ICSBP-/- compared with WT mice. These findings reflected a significantly reduced frequency of skin-derived CD11c+CD40hi DCs and their slower turnover rate in lymph nodes of ICSBP-/- mice. In this regard, several authors have reported that skin-derived DCs display the slowest turnover among the DC populations present in cutaneous LNs, reflecting a long life spent within the skin by both dermal and epidermal DCs.27,32 In addition, there is evidence indicating that the vast majority of skin-resident DCs are in fact sessile and hence need long periods to migrate out of the skin.27,32,40 In this light, although the reason for the reduction in number and for the slower turnover observed in CD11c+CD40hi DCs of ICSBP-/- mice remains to be uncovered, we can argue that ICSBP-/- mice may display a defective renewal of DCs in the skin. This might be due in part to an impaired recruitment of LCs from the BM to the skin, as suggested by the lower frequency of LCs in the epidermis of ICSBP-/- mice. In addition, ICSBP-/- skin DCs may display a defective response to chemotactic signals driving their homing to LNs in the steady state, as indicated by their lack of response to 6Ckine in skin explant cultures. However, we cannot rule out the possibility that skin DCs of ICSBP-/- mice might have a longer life span, since ICSBP is an important factor controlling apoptosis in hematopoietic cells.18,41
Another important finding reported in this paper is that ICSBP-/- mice display a profound impairment in skin DC migration during inflammation. After skin sensitization, induced by topical application of FITC, ICSBP-/- mice displayed defective migration of Ag-bearing DCs from the sensitized skin to the regional LNs. In fact, unlike WT mice, FITC+ DCs were not detected in LNs of knockout mice 24 hours after treatment with FITC. These findings correlated with an absence of mobilization from skin of ICSBP-/- mice, as revealed by the frequency of DCs in skin explants of FITC-sensitized versus unsensitized mice. Notably, FITC+CD11c+ cells started to accumulate in LNs of ICSBP-/- mice with 1 day of delay compared with WT animals, suggesting that ICSBP-/- mice were defective at generating a rapid response to the stimulus. The prompt mobilization of DCs from skin represents a crucial event in the initiation of the immune response to environmental insults.2,9 Thus, the delayed mobility of ICSBP-/- skin DCs may account for their defective induction of T-cell–mediated responses. Indeed, ICSBP-/- mice failed to induce CHS responses after skin sensitization with FITC. CHS is a dendritic cell–mediated, T-cell–dependent immune response to skin inflammation, which is elicitated by re-exposing the skin to the sensitizing hapten.42 The magnitude of the CHS response is indicative of the efficacy of Ag presentation during the sensitization phase. Although the absence of a CHS response strongly correlates with the defective migration of Ag-bearing DCs in ICSBP-/- mice, other factors may also be involved, since some DCs were still able to reach the LNs in ICSBP-/- mice. In particular, as CHS responses represent a Th-1 type of immune response largely depending on DC-derived IL-12 production,43,44 the impaired expression of IL-12 by ICSBP-/- DCs may represent a key event for the lack of CHS elicitation. In this respect, the defect in IL-12 expression by immune cells in ICSBP-/- mice has been correlated with impaired priming of Th-1 immune responses against several pathogens.45,46
BM-derived DCs generated in vitro in presence of GM-CSF displayed typical DC features and differentiated preferentially into myeloid DCs.35,36 We found that even though CD11c+ cells could be generated from BM cultures of ICSBP-/- mice, these cells exhibited a defective developmental pathway and an impaired response to chemokines that control DC migration at different maturation stages. These findings suggest that ICSBP may control the development of BM DCs at different stages of differentiation and are consistent with in vivo studies showing that myeloid DC subsets are present in normal numbers in lymphoid organs of ICSBP-/- mice but display defective responses to activation signals.19,21 The concept that ICSBP controls the maturation of myeloid DCs is also supported by our findings that BM DCs from ICSBP-/- mice still display an immature phenotype after 7 days of culture. This was revealed not only by the absence of CD40 expression and the low levels of CD80, CD86, and MHC class II molecules but also by the impaired expression of IL-12p40. In this regard, it has been reported that transcription of both IL-12p35 and IL-12p40 mRNAs occurs at a mature stage of BM DC development.37 Similarly, IL-12p70 secretion correlates with the functional maturation of splenic DC lines, thus indicating that IL-12 expression is associated with a mature DC phenotype.47 Of note, ICSBP has been shown to regulate IL-12 expression in splenic DCs as well as in BM DCs generated in presence of Flt3-L.19,22 Therefore, the lack of induction of IL-12p40 expression during the final stages of differentiation of BM DCs from ICSBP-/- mice may represent a critical aspect for the correct maturation of DCs and their ability to prime T-cell responses, such as CHS.
Seven-day cultured BM DCs from ICSBP-/- mice were also found to exhibit very low levels of CCR7 when compared with DCs from control animals. Accordingly, these DCs failed to respond to 6Ckine and showed an impaired migration toward MIP-3β. In addition, ICSBP-/- BM DCs displayed a selective, early reduction of CCR6 expression and failed to migrate in response to MIP-3α. In vivo, MIP-3β and 6Ckine are mainly expressed within the T-cell areas of inflamed LNs and play a pivotal role in the homing of maturing DCs from peripheral tissues to the site of Ag presentation, as a consequence of CCR7 up-regulation on the membrane of mature DCs.10,11 In contrast, MIP-3α is expressed at the site of Ag entry and is the major chemokine induced in peripheral tissues during inflammation, driving the recruitment of immature DCs via CCR6.8 In this regard, it has been suggested that CCR6 expression by LCs and their precursors, along with secretion of MIP-3α by surrounding keratinocytes, may represent a crucial event in LC mobilization into both normal and inflamed skin.8,48
Taken together, our results show that ICSBP plays a key role in the regulation of skin DC trafficking under both steady-state and inflammatory conditions. We suggest that ICSBP may regulate this process through the control of functional maturation of skin DCs. Very recently, it has been shown that the transcription factor Id2 affects the lineage choice of DC progenitors leading to lack of LCs and reduced CD8α+ DCs in Id2-/- mice.49 Similarly, we suggest that ICSBP signaling pathway may direct the differentiation of DC populations from BM precursors at different developmental stages and through selective regulation of chemokine receptor expression, thereby affecting DC trafficking and, as a consequence, the generation of appropriately directed T-cell–mediated immunity.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-09-3007.
Supported by grants from the Italian Association for Cancer Research (Project AIRC no. F89) and the Italian Ministry of Health (special project entitled “Dendritic Cells and Type I IFN”).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs David F. Tough and Arun T. Kamath for critical reading of the manuscript.