Abstract
B cells of chronic lymphocytic leukemia (CLL) are long-lived in vivo, possibly because of defects in apoptosis. We investigated BL22, an immunotoxin composed of the Fv portion of an anti-CD22 antibody fused to a 38-kDa Pseudomonas exotoxin-A fragment. B cells from 22 patients with CLL were immunomagnetically enriched (96% purity) and were cultured with BL22 or an immunotoxin that does not recognize hematopoietic cells. The antileukemic activity of BL22 was correlated with CD22 expression, as determined by flow cytometry. BL22 induced caspase-9 and caspase-3 activation, poly(adenosine diphosphate [ADP]-ribose)polymerase (PARP) cleavage, DNA fragmentation, and membrane flipping. Cell death was associated with the loss of mitochondrial membrane potential and the down-regulation of Mcl-1 and X-chromosomal inhibitor of apoptosis protein (XIAP). Furthermore, BL22 induced a proapoptotic 18-kDa Bax protein and conformational changes of Bax. Z-VAD.fmk abrogated apoptosis, confirming that cell death was executed by caspases. Conversely, interleukin-4, a survival factor, inhibited spontaneous death in culture but failed to prevent immunotoxin-induced apoptosis. BL22 cytotoxicity was markedly enhanced when combined with anticancer drugs including vincristine. We also investigated HA22, a newly engineered immunotoxin, in which BL22 residues are mutated to improve target binding. HA22 was more active than BL22. In conclusion, these immunotoxins induce caspase-mediated apoptosis involving mitochondrial damage. Combination with chemotherapy is expected to improve the efficacy of immunotoxin treatment. (Blood. 2004;103:2718-2726)
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent form of leukemia in the Western hemisphere; it accounts for an estimated 8100 new cases and 4800 deaths annually in the United States.1 Ninety-five percent of CLL cases in the United States are of B-cell origin.2 Patients require treatment if normal hematopoiesis is impaired by the infiltration of leukemic B cells into the bone marrow or if the disease causes disorders of the immune system. Although chemotherapy, such as with nucleoside analogs, can induce remission in CLL, few patients are cured even with high-dose chemotherapy and stem cell support.3,4
Compared with those of several other non-Hodgkin lymphomas, CLL B cells have been found to be relatively resistant to apoptosis in peripheral blood and to the induction of programmed cell death by cytotoxic agents.5,6 Furthermore, most CLL cells in the peripheral blood appear to be quiescent and arrested in the G0/G1 phase of the cell cycle because of the altered regulation of early cell cycle progression.7-10 Thus, high leukocyte counts result from prolonged survival rather than from accelerated proliferation.11
In addition to chemotherapeutic agents, targeted therapies with monoclonal antibodies and immunotoxins have recently demonstrated clinical efficacy in non-Hodgkin lymphomas.12-14 Immunotoxins consist of a toxic protein, such as diphtheria toxin, ricin, or Pseudomonas exotoxin, that causes cell death when internalized into cells, and a monoclonal antibody or an antibody fragment that recognizes a certain cell type.15,16 An immunotoxin designated BL22 or RFB4(dsFv)-PE38, which is directed toward the CD22 antigen on B-lymphocytes, is highly active in hairy cell leukemia; it induced complete remissions in 11 of 16 heavily pretreated patients.17 In preclinical testing, the immunotoxin displayed high cytotoxicity to cells that express high levels of CD22.18 In B-cell CLL (B-CLL), however, CD22 is expressed only at low to moderate levels.19 Nonetheless, antileukemic activity has been observed in samples from CLL patients.20
BL22 is a recombinant protein composed of the variable portion of monoclonal antibody RFB4, which binds to the CD22 glycoprotein on the surface of B cells, and of PE38, a truncated Pseudomonas exotoxin derivative.21 Lacking the cell-binding domain of Pseudomonas exotoxin, PE38 is only incorporated into target cells to which the Fv domain binds.21 To exert its cytotoxicity, internalization and processing of the toxin within its translocation domain are required,22 followed by binding of the 35-kDa carboxy-terminus of the toxin intracellularly to the KDEL receptor that carries it to the endoplasmic reticulum.23 Finally, the toxin is translocated to the cytosol, where its adenosine diphosphate (ADP)-ribosylating enzyme domain inactivates elongation factor 2 (EF-2), thereby inhibiting protein synthesis.24,25 Cell death mediated by PE38 is often facilitated by apoptosis, though immunotoxins can kill cells without inducing caspase-dependent programmed cell death, and other mechanisms may play a role in certain cell types.26,27
Multiple steps are involved in the mode of action of PE38-based immunotoxins. Although binding of the immunotoxin to cellular target structures is required, the number of binding sites for the respective immunotoxin is not necessarily directly correlated with the cytotoxic response. This has been found in an anti-CD25 immunotoxin, which is less active in CLL than in hairy cell leukemia even though CLL cells express higher numbers of target molecules on their surfaces.28
In the present work, we investigated whether the immunotoxin BL22 can overcome resistance to apoptosis in CLL. We found that CLL cells die from caspase-mediated programmed cell death, characterized by DNA fragmentation and membrane flipping, involving the loss of mitochondrial membrane potential. The antileukemic activity of BL22 is maintained in the presence of an antiapoptotic survival factor for CLL cells29 and is further enhanced in combination with certain anticancer drugs or by the introduction of mutations that increase affinity to the CD22 target antigen.
Patients, materials, and methods
Isolation of B-CLL samples by immunomagnetic separation
After informed consent was obtained, peripheral blood was collected from patients with B-CLL confirmed according to morphologic and immunophenotypic criteria. Patient characteristics are shown in Table 1. The ethics committee of the Technische Universität (Munich, Germany) approved the analysis of cell samples from B-CLL patients. Patients were either untreated or had not received cytoreductive chemotherapy for at least 3 months before blood collection. By the time of analysis, all patients were clinically stable and free of infectious complications.
Enrichment of B-CLL cells by immunomagnetic sorting has been described.30 In brief, mononuclear cells were first enriched by density gradient centrifugation over a Ficoll-Hypaque layer, and then T-lymphocytes and monocytes were depleted with immunomagnetic microbeads. As assessed by flow cytometry, the content of CD19+ B cells was greater than 96% in all immunoseparated samples. Cells were used immediately for further experiments or were resuspended in fetal bovine serum (FBS) with 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen until use. Control experiments revealed similar results when fresh cells were compared with frozen samples from the same patients (not shown).
Detection of CD22 target antigen
Cells were washed in phosphate-buffered saline (PBS) containing 2% FBS (Biochrom, Berlin, Germany). They were then incubated for 30 minutes at 4°C with saturating amounts of fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies to CD22 (Immunotech, Marseille, France) or with equal amounts of a nonbinding, isotype-identical control antibody (Immunotech), followed by washing with PBS containing 2% FBS. Fluorescence intensities were analyzed with a Coulter Epics XL cytofluorometer (Krefeld, Germany) using WinMDI 2.8 FACS software (from J. Trotter, Scripps Institute, San Diego, CA). A level of fluorescence intensity was identified, such that less than 2% of cells stained with an isotype control antibody displayed fluorescence intensities above this level (threshold). The percentage of CLL cells whose fluorescence was above this threshold when stained with anti-CD22 antibodies was recorded.
Preparation of immunotoxins
Development and production of the disulfide-linked, recombinant immunotoxin BL22 (RFB4(dsFv)-PE38), which reacts with the CD22 antigen, has been described.21 Engineering of a mutated anti-CD22 single-chain immunotoxin with an increased affinity for CD22 has been reported.31 HA22, a disulfide-linked immunotoxin containing the respective mutations in its variable region, was constructed according to the method described.32 LMB-9 (B3(dsFv)-PE38), a disulfide-linked immunotoxin specific for a LewisY antigen, which is not present on hematopoietic cells, was used as a control.32,33
Culture conditions
Leukemic B cells were cultured in RPMI 1640 medium, supplemented with 10% FBS, 50 IU/mL penicillin/streptomycin, 1 mM sodium pyruvate, 2 mM l-glutamine, 20 μg/mL l-asparagine, 0.05 mM 2-mercaptoethanol, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and minimum essential medium (MEM) nonessential amino acids 0.7× (all from Biochrom, Berlin, Germany). All tissue-culture experiments were performed in a fully humidified atmosphere with 5% CO2 and at 37°C. Cells were plated at 1 × 106 cells in a total volume of 1 mL in 24-well dishes and were grown for 1 to 3 days. Immunotoxins were added at indicated concentrations. In some experiments, the medium was supplemented with interleukin-4 (IL-4) at 10 ng/mL (R&D Systems, Wiesbaden, Germany) or with fludarabine, vincristine, or doxorubicin (all from Sigma, Deisenhofen, Germany).
Immunoblotting
Cells grown in the presence or in the absence of immunotoxin were harvested from cultures and were washed twice with PBS. They were then lysed in ice-cold 10 mM Tris-HCl buffer (pH 7.4) containing 5 mM EDTA (ethylenediaminetetraacetic acid), 130 mM NaCl, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium vanadate, and 10 mg/mL each phenanthrolene, aprotinin, leupeptin, and pepstatin. Cell lysates were spun at 12 000 rpm for 20 minutes, and supernatants were collected. After determination was made of the protein content with a commercially available assay (Bio-Rad, Richmond, CA), 50 μg protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and was transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore Schwalbach, Germany). They were hybridized with antibodies to PARP and Bax (purchased from BD PharMingen, San Diego, CA), to Mcl-1 (from Santa Cruz Biotechnology, Santa Cruz, CA), to X-chromosomal inhibitor of apoptosis protein (XIAP) and Bcl-2 (from BD Biosciences Transduction Laboratory, San Diego, CA), or to actin (from Sigma). Antibodies to caspase-8 and caspase-9 were from Cell Signaling (Frankfurt, Germany). Blots were developed using Super Signal chemoluminescent substrates (Pierce, KMF GmbH, St Augustin, Germany).
Analysis of fragmented DNA and of membrane flipping
The amount of fragmented DNA was assessed by a TdT-mediated dUTP nick-end labeling (TUNEL) assay, as reported.34 Incorporation of fluorescein-labeled dUTP into DNA strand breaks was detected by flow cytometry. Exposure of phosphatidylserine (PS) on the outside of the plasma membrane was analyzed by flow cytometry, as described.10 In brief, cells were stained with FITC-labeled monoclonal antibodies to Annexin V and were counterstained with propidium iodide (PI). Numbers of viable cells (VCs), identified as negative for Annexin and PI, varied from sample to sample because of spontaneous apoptosis in culture. To calculate the induction of apoptosis by a toxin, the number of viable cells in the presence of toxin (VCtoxin) was corrected for the number of viable cells in medium without toxin (VCmedium) according to the equation (VCmedium - VCtoxin)/VCmedium × 100.
Investigation of mitochondrial membrane potential
Mitochondrial membrane potential, Δψm, was studied by flow cytometric analysis of the fluorescence of 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3)), as reported.10
Flow cytometric detection of intracellular proteins
Cells were fixed and permeabilized using Fix & Perm kit (Caltag, Burlingame, CA) for 15 minutes at room temperature. Activated caspase-3 was detected by flow cytometry with an FITC-conjugated monoclonal antibody that fails to recognize the inactive enzyme (clone C92-605; BD Biosciences) and was compared with an appropriate isotype control (BD Biosciences). Conformational change of Bax was detected using a conformation-specific antibody, YTH-6A7 (Trevigen, Gaithersburg, MD).
Results
Expression of CD22 and response to treatment with the immunotoxin BL22
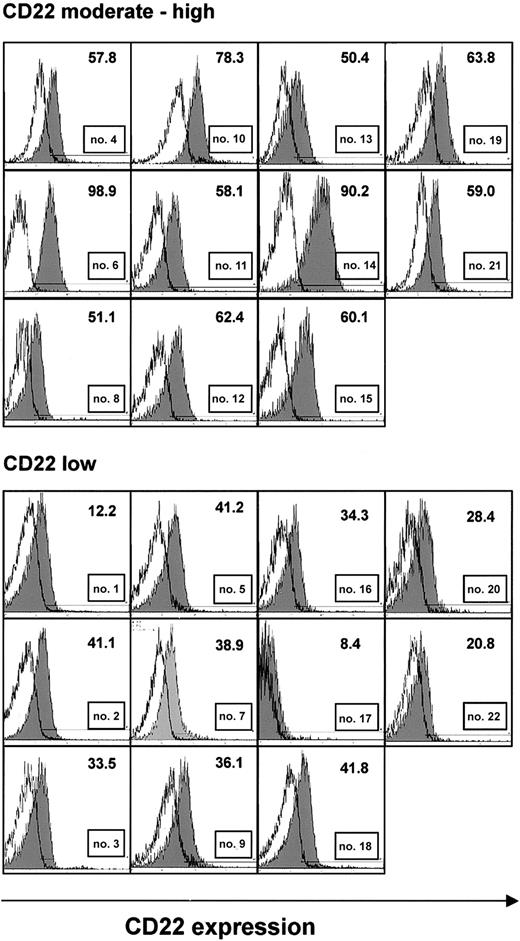
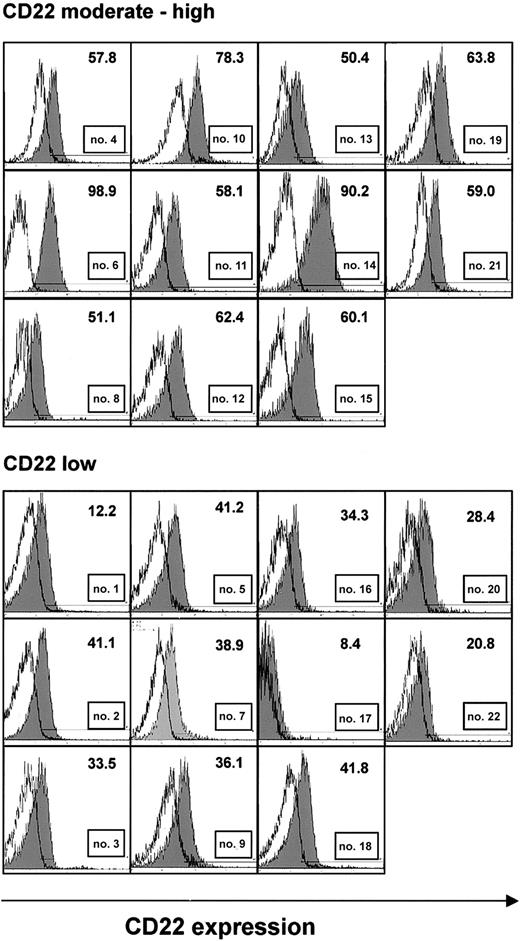
Samples from 22 CLL patients, immunoseparated to more than 96% purity based on the content of CD19+ B cells, were analyzed by flow cytometry for expression of the CD22 antigen. CD22 levels were generally moderate to low (Figure 1). Expression was particularly low in 11 samples in which less than 50% of CLL cells displayed fluorescence intensities above the levels of isotype control (Figure 1, lower panel). There was no correlation between CD22 expression and age, sex, Binet stage, or leukocyte count (Table 1).
CD22 expression in purified B-CLL cells. CLL cells were immunoseparated to more than 96% purity. They were stained with FITC-labeled monoclonal antibodies to CD22 or a nonbinding control and were analyzed by flow cytometry. Histograms show fluorescence of an anti-CD22 antibody (shaded), superimposed with that of an isotype control (solid line) from all 22 patients. A threshold level of fluorescence intensity was identified (shown as a horizontal line), such that fewer than 2% of cells stained with the control antibody displayed fluorescence above this level. The number of cells with anti-CD22 fluorescence above this threshold is indicated. Samples, in which less than 50% of CLL cells had anti-CD22 fluorescence above the threshold, were considered low expressing.
CD22 expression in purified B-CLL cells. CLL cells were immunoseparated to more than 96% purity. They were stained with FITC-labeled monoclonal antibodies to CD22 or a nonbinding control and were analyzed by flow cytometry. Histograms show fluorescence of an anti-CD22 antibody (shaded), superimposed with that of an isotype control (solid line) from all 22 patients. A threshold level of fluorescence intensity was identified (shown as a horizontal line), such that fewer than 2% of cells stained with the control antibody displayed fluorescence above this level. The number of cells with anti-CD22 fluorescence above this threshold is indicated. Samples, in which less than 50% of CLL cells had anti-CD22 fluorescence above the threshold, were considered low expressing.
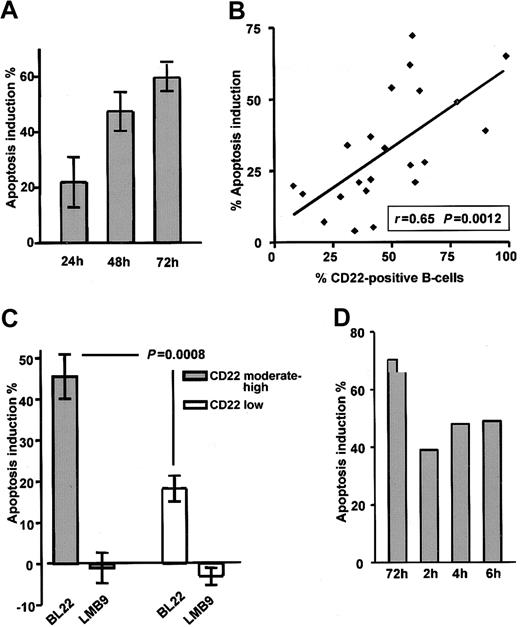
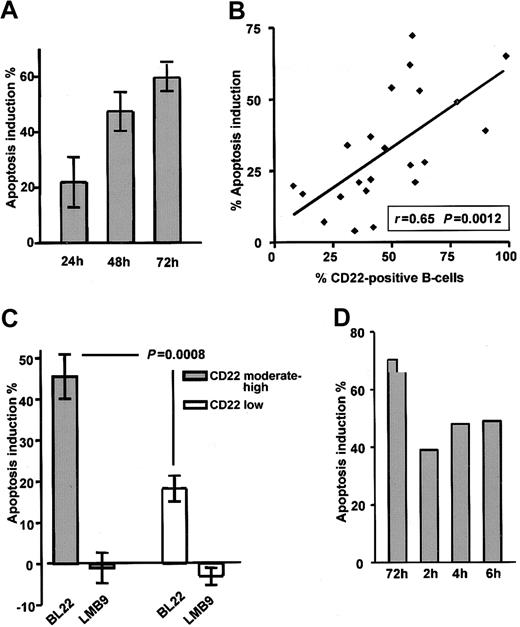
CLL cells were cultured in the presence of the immunotoxin BL22 at 1000 ng/mL. As a typical feature of programmed cell death, flipping of the plasma membrane, which leads to the exposure of PS, was analyzed by Annexin V staining. BL22 induced apoptosis in a time-dependent fashion (Figure 2A). Numbers of apoptotic cells increased from 24 to 72 hours. Based on these results, a time period of 72 hours was chosen for all further experiments. Results obtained from the Annexin V assay were validated with those of a colorimetric MTT assay32 ; close correlation (r = 0.85) was observed (data not shown). It should be noted that in addition to the toxicity of BL22, some spontaneous cell death was observed in all cultured CLL samples. Spontaneous death of cultured CLL cells was unaffected by the immunotoxin LMB-9, which reacts with adenocarcinomas but not with hematopoietic cells (negative control). Numbers shown are corrected for the percentages of apoptotic cells in the absence of toxin. However, 3 additional patient samples had to be excluded from all analyses because spontaneous apoptosis exceeded 70%.
Death of CLL cells depends on exposure time and CD22 expression. (A) To determine the time-course of cytotoxicity, CLL cells were grown for indicated periods in the presence of 1000 ng/mL BL22 or LMB-9 or in the absence of immunotoxin. Cells were then stained with Annexin V/PI and were analyzed by flow cytometry. To calculate apoptosis induction by BL22, the percentage of spontaneously apoptotic cells in the absence of toxin was subtracted as described in “Patients, materials, and methods.” Results represent the means of 3 individual patient samples. (B) Comparison of apoptosis induction by 1000 ng/mL BL22 for 72 hours and CD22 expression in 22 different patient samples. CD22 was determined as described in the legend to Figure 1. (C) Apoptosis induction of 11 samples expressing CD22 at moderate to high levels (containing more than 50% CD22+ cells) compared with 11 low-expressing samples (containing less than 50% CD22+ cells). Results represent means ± SEM. The Mann-Whitney U test was used for statistical analysis. (D) Effect of short-term exposure to immunotoxin. CLL cells were incubated for 2, 4, or 6 hours or continuously for 72 hours in the presence of BL22 at 1000 ng/mL. CLL cells exposed to BL22 for short periods were harvested, washed 3 times, and transferred to fresh medium without immunotoxin. After a total culture period of 72 hours, cells were analyzed for apoptosis induction by flow cytometry using Annexin V/PI. Shown are results from a representative experiment; a second experiment gave similar results.
Death of CLL cells depends on exposure time and CD22 expression. (A) To determine the time-course of cytotoxicity, CLL cells were grown for indicated periods in the presence of 1000 ng/mL BL22 or LMB-9 or in the absence of immunotoxin. Cells were then stained with Annexin V/PI and were analyzed by flow cytometry. To calculate apoptosis induction by BL22, the percentage of spontaneously apoptotic cells in the absence of toxin was subtracted as described in “Patients, materials, and methods.” Results represent the means of 3 individual patient samples. (B) Comparison of apoptosis induction by 1000 ng/mL BL22 for 72 hours and CD22 expression in 22 different patient samples. CD22 was determined as described in the legend to Figure 1. (C) Apoptosis induction of 11 samples expressing CD22 at moderate to high levels (containing more than 50% CD22+ cells) compared with 11 low-expressing samples (containing less than 50% CD22+ cells). Results represent means ± SEM. The Mann-Whitney U test was used for statistical analysis. (D) Effect of short-term exposure to immunotoxin. CLL cells were incubated for 2, 4, or 6 hours or continuously for 72 hours in the presence of BL22 at 1000 ng/mL. CLL cells exposed to BL22 for short periods were harvested, washed 3 times, and transferred to fresh medium without immunotoxin. After a total culture period of 72 hours, cells were analyzed for apoptosis induction by flow cytometry using Annexin V/PI. Shown are results from a representative experiment; a second experiment gave similar results.
The antileukemic efficacy of BL22 strongly depended on the presence of the CD22 target antigen. Good correlation (r = 0.65; P = .0012) was found in 22 samples between CD22 expression and apoptosis induction after treatment with BL22 (Figure 2B). Moreover, as shown in Figure 2C, apoptosis induction was significantly higher in the group of 11 CLL samples expressing CD22 at moderate levels (more than 50% CD22+ cells) than in 11 low-expressing samples (P = .0008). The immunotoxin LMB-9 displayed no activity in either group. Based on these experiments, it was determined that flow cytometric analysis of CD22 expression was suitable to predict in vitro response to treatment with BL22. It should be noted, however, that normal B-lymphocytes are also susceptible to BL22 toxicity because they express high numbers of CD22 target molecules (data not shown).
Although drug administration by continuous infusion is feasible in the clinic, shorter time periods are more practical. Therefore, we investigated whether CLL cells also die when exposed to the immunotoxin for shorter periods. To this end, cells were cultured in the presence of BL22 for 2, 4, or 6 hours. Cells were then washed and transferred to medium without toxin. Apoptosis induction was assessed in all samples after a total culture period of 72 hours. As displayed in Figure 2D, short-term treatment for as little as 2 hours induced apoptosis in a substantial number of CLL cells even though continuous exposure was more effective.
BL22 kills CLL cells through caspase-mediated apoptosis
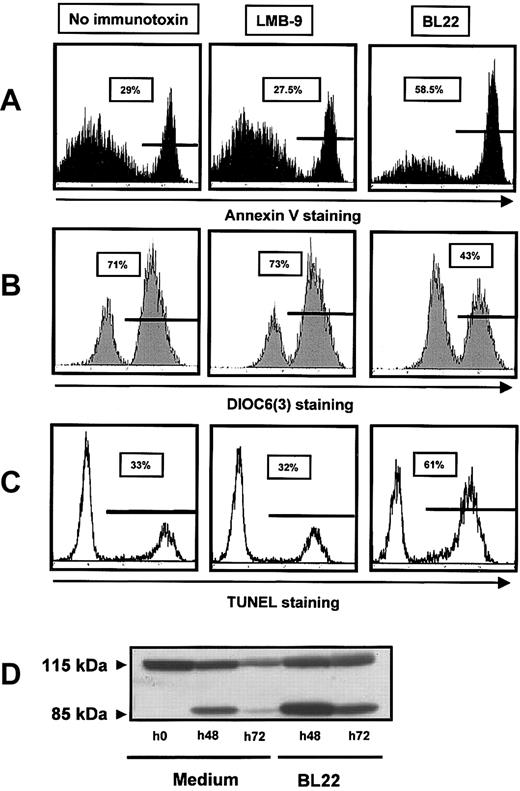
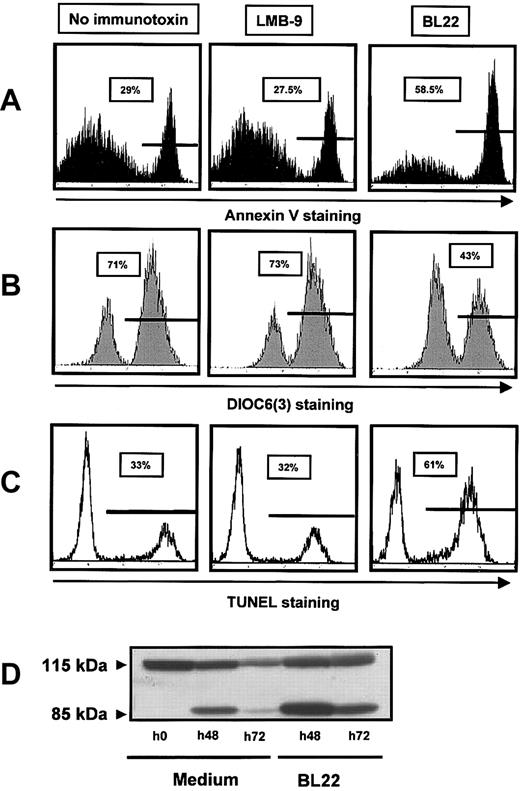
To characterize the cytotoxic action of the immunotoxin, we investigated caspase activation, induction of DNA fragmentation, and changes in mitochondrial membrane potential. DNA-strand breaks were assessed using TUNEL assay. They were markedly increased in BL22-treated cells compared with untreated or LMB-9-treated cells (Figure 3A). Numbers of apoptotic cells detected with Annexin V staining were almost identical with those obtained with the TUNEL assay (Figure 3C). Next we investigated mitochondrial transmembrane potential Δψm by staining with a fluorescent dye, DiOC6(3). As shown in Figure 3B, incubation with BL22 caused a distinct reduction in the number of cells with high-level DiOC6(3) fluorescence. BL22-induced cell death is, therefore, associated with damage to the mitochondrial membrane.
BL22 cytotoxicity involves DNA-strand breaks, PARP cleavage, and damage to mitochondria. CLL cells were cultured for 72 hours in the presence of BL22 or LMB-9 at 1000 ng/mL or in the absence of immunotoxin. After staining with fluorescent compounds, cells were analyzed by flow cytometry. (A) Cells were stained for Annexin V, as described in the legend to Figure 2. (B) For analysis of the mitochondrial membrane potential Δψm, cells were incubated with DiOC6(3) for 30 minutes. Results from 1 representative experiment are displayed; 2 additional experiments gave similar results. (C) DNA-strand breaks were assessed using TUNEL assay, as described in “Patients, materials, and methods.” (D) CLL cells were grown for indicated periods in the presence of BL22 or in medium without immunotoxin. Cell lysates containing 50 μg protein were loaded into each lane, and cleavage of PARP was analyzed by immunoblotting, as described in “Patients, materials, and methods.” An additional experiment gave comparable results.
BL22 cytotoxicity involves DNA-strand breaks, PARP cleavage, and damage to mitochondria. CLL cells were cultured for 72 hours in the presence of BL22 or LMB-9 at 1000 ng/mL or in the absence of immunotoxin. After staining with fluorescent compounds, cells were analyzed by flow cytometry. (A) Cells were stained for Annexin V, as described in the legend to Figure 2. (B) For analysis of the mitochondrial membrane potential Δψm, cells were incubated with DiOC6(3) for 30 minutes. Results from 1 representative experiment are displayed; 2 additional experiments gave similar results. (C) DNA-strand breaks were assessed using TUNEL assay, as described in “Patients, materials, and methods.” (D) CLL cells were grown for indicated periods in the presence of BL22 or in medium without immunotoxin. Cell lysates containing 50 μg protein were loaded into each lane, and cleavage of PARP was analyzed by immunoblotting, as described in “Patients, materials, and methods.” An additional experiment gave comparable results.
To further elucidate the mode of cell death, we analyzed cleavage of the DNA repair enzyme PARP, a substrate of caspases, and activation of caspase-3. As shown in Figure 3D, only the intact PARP protein of 115 kDa was seen in freshly isolated CLL cells. A shorter product of 85 kDa, in addition to full-length PARP, was observed after 48 hours, consistent with spontaneous cell death. Spontaneous cell death in culture was also observed with other assays (Figure 3A-C). However, the amount of cleaved PARP was markedly increased in the presence of BL22 (Figure 3D). After 72 hours of culture, we found less full-length and cleaved PARP, possibly because of protein degradation with increasing numbers of dying cells.
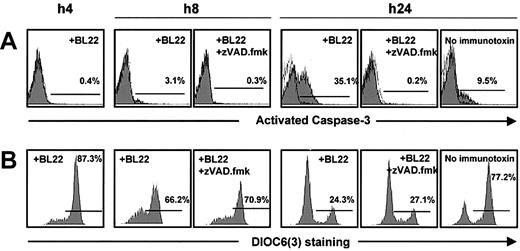
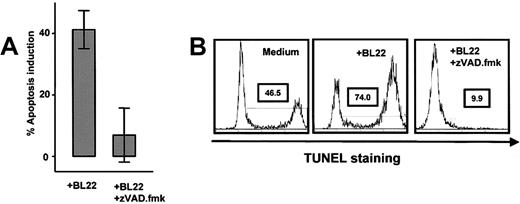
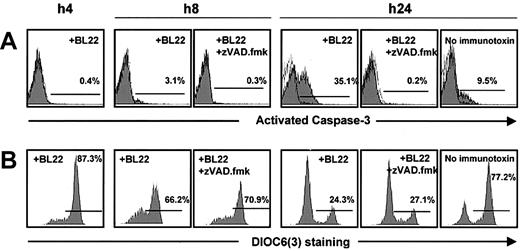
Activation of caspase-3 in BL22-treated B-CLL cells was noted after 24 hours, whereas little activated caspase-3 was detected at earlier time points (Figure 4A). The antibody used for this assay failed to recognize the inactive form. To confirm caspase-3 activation, Z-VAD.fmk, an inhibitor of caspases, was added to the cultures. When BL22 was combined with Z-VAD.fmk, the cell population with activated caspase-3 was almost completely lost (Figure 4A). Interestingly, Z-VAD.fmk inhibited not only BL22-induced apoptosis but also spontaneous apoptosis in CLL cells cultured in the absence of immunotoxin (Figure 4A).
Time-course of mitochondrial damage and caspase-3 activation. B-CLL cells from one patient were cultured with 1000 ng/mL BL22 in the presence or absence of 100 μM Z-VAD.fmk, or, as a control, without immunotoxin. Cells were harvested after indicated time periods and were analyzed by flow cytometry. (A) After permeabilization of CLL cells, intracytoplasmic staining was performed with monoclonal antibody against the active form of caspase-3. (B) For analysis of the mitochondrial membrane potential Δψm, cells were incubated with DiOC6(3) for 30 minutes.
Time-course of mitochondrial damage and caspase-3 activation. B-CLL cells from one patient were cultured with 1000 ng/mL BL22 in the presence or absence of 100 μM Z-VAD.fmk, or, as a control, without immunotoxin. Cells were harvested after indicated time periods and were analyzed by flow cytometry. (A) After permeabilization of CLL cells, intracytoplasmic staining was performed with monoclonal antibody against the active form of caspase-3. (B) For analysis of the mitochondrial membrane potential Δψm, cells were incubated with DiOC6(3) for 30 minutes.
Caspase-3 activation was preceded by reductions in the numbers of cells with intact mitochondrial potential, which was already noticed after 8 hours (Figure 4B). Thus, mitochondrial damage appeared to initiate caspase-3 activation rather than being an epiphenomenon during the execution of apoptosis. This idea is further confirmed by control experiments in which cells were treated with BL22 in combination with Z-VAD.fmk. The inhibitor reversed caspase-3 activation but failed to circumvent the loss of mitochondrial potential (Figure 4). Hence, damage to the mitochondrial membrane is an upstream event that precedes caspase-3 activation.
Conversely, late events in the apoptotic cascade, such as membrane flipping and DNA strand breaks, were inhibited when Z-VAD.fmk was added to the cultures. No apoptotic cells were recognized with Annexin V or TUNEL assays in BL22-treated cells if Z-VAD.fmk was added (Figure 5). In summary, the immunotoxin exerts its toxicity to CLL cells by caspase-dependent apoptosis, which involves disruption of the mitochondrial membrane and DNA fragmentation.
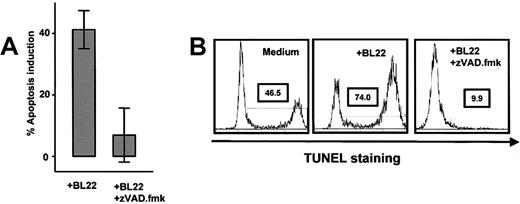
Effects of caspase inhibition. (A) Purified B-CLL cells from 8 different samples were cultured for 72 hours with 1000 ng/mL BL22 in the presence or absence of 100 μM Z-VAD.fmk. Apoptosis induction was determined by Annexin V staining as described in “Patients, materials, and methods.” Results are presented as means ± SEM. (B) TUNEL staining was performed in 3 different samples cultured with either BL22 ± Z-VAD.fmk or, as a control, medium without supplementation. Shown is a representative experiment. Similar results were obtained in 2 additional experiments.
Effects of caspase inhibition. (A) Purified B-CLL cells from 8 different samples were cultured for 72 hours with 1000 ng/mL BL22 in the presence or absence of 100 μM Z-VAD.fmk. Apoptosis induction was determined by Annexin V staining as described in “Patients, materials, and methods.” Results are presented as means ± SEM. (B) TUNEL staining was performed in 3 different samples cultured with either BL22 ± Z-VAD.fmk or, as a control, medium without supplementation. Shown is a representative experiment. Similar results were obtained in 2 additional experiments.
The critical role of the intrinsic, mitochondria-dependent pathway of apoptosis is also supported by flow cytometric analysis of the CD95 death receptor. No up-regulation of CD95 was observed in BL22-treated CLL cells (data not shown).
To further characterize the apoptotic pathways involved, we analyzed procaspase-8 and procaspase-9. Levels of procaspase-9 were clearly reduced, consistent with activation of this caspase (Figure 6A). In contrast, levels of procaspase-8 were decreased to a lesser extent. In these experiments, a small amount of cleaved enzyme was detected, presumably corresponding to the active caspase-8 (Figure 6A). Thus, the intrinsic, caspase-9-dependent pathway appeared to be mainly involved in programmed cell death, though caspase-8 activation might also have contributed to B-CLL cell death.
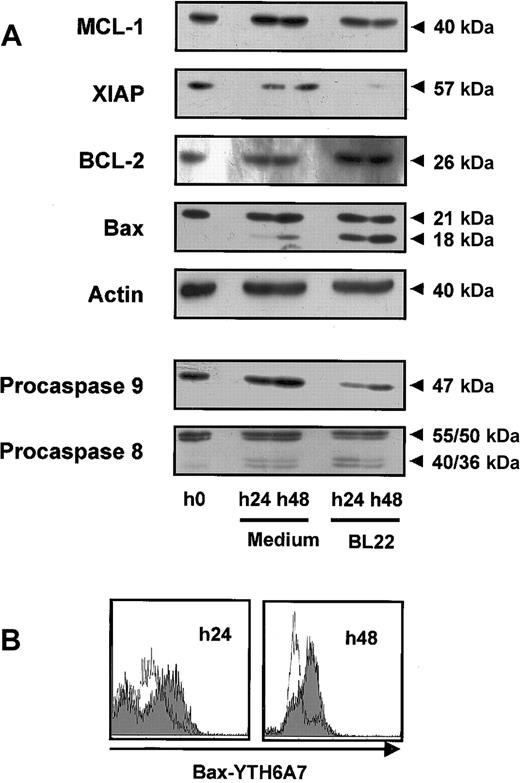
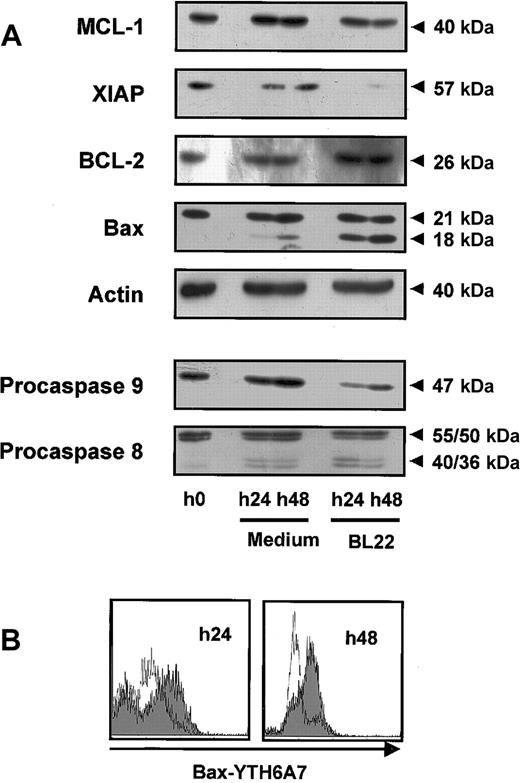
Mitochondrial survival proteins and procaspases in BL22-treated B-CLL cells. CLL cells were cultured for indicated periods in the presence or absence of 1000 ng/mL BL22. (A) CLL cells were harvested from cultures and lysed. Lysates (50 μg/mL) were separated by SDS-PAGE and were transferred to membranes. Membranes were hybridized with antibodies to proapoptotic or antiapoptotic proteins, to caspase-8 and -9, or to actin (loading control). Blots were developed using chemoluminescent substrates. Approximate sizes of bands are indicated. Shown are results from a representative experiment; a second experiment gave comparable results. (B) CLL cells were permeabilized and stained with antibody YTH-6A7, which recognized conformational changes of Bax but failed to detect the unchanged Bax protein. Cells were analyzed by flow cytometry. Fluorescence intensities of BL22-treated CLL cells are shown as a gray area; intensities of untreated cells are overlaid as a thin line. Shown are results of a representative experiment; 2 additional patient samples gave similar results.
Mitochondrial survival proteins and procaspases in BL22-treated B-CLL cells. CLL cells were cultured for indicated periods in the presence or absence of 1000 ng/mL BL22. (A) CLL cells were harvested from cultures and lysed. Lysates (50 μg/mL) were separated by SDS-PAGE and were transferred to membranes. Membranes were hybridized with antibodies to proapoptotic or antiapoptotic proteins, to caspase-8 and -9, or to actin (loading control). Blots were developed using chemoluminescent substrates. Approximate sizes of bands are indicated. Shown are results from a representative experiment; a second experiment gave comparable results. (B) CLL cells were permeabilized and stained with antibody YTH-6A7, which recognized conformational changes of Bax but failed to detect the unchanged Bax protein. Cells were analyzed by flow cytometry. Fluorescence intensities of BL22-treated CLL cells are shown as a gray area; intensities of untreated cells are overlaid as a thin line. Shown are results of a representative experiment; 2 additional patient samples gave similar results.
Immunotoxin treatment changes the levels of mitochondrial survival proteins
Members of the Bcl-2 family regulate the induction of programmed cell death at the mitochondrial level. Using Western blot analysis, we found that exposure to BL22 led to reduced levels of the antiapoptotic Mcl-1 protein while Bcl-2 levels remained unaffected (Figure 6A). The decrease of Mcl-1 levels was noted after 24 hours and was enhanced after 48 hours. Furthermore, XIAP expression was diminished with BL22 treatment.
We also investigated expression of the proapoptotic protein Bax. Western blot analysis of Bax revealed a smaller band of approximately 18 kDa in addition to a 21-kDa band when cells were exposed to BL22. In contrast, only minute amounts of the smaller protein were detected in untreated cells. Staining with a monoclonal antibody specific for actin confirmed that the observed differences in protein expression were not caused by different amounts of protein per lane.
To further characterize the role of Bax, intracellular flow cytometry was performed with the antibody YTH-6A7, which is directed against the NH2-terminal region of Bax. This antibody recognizes only a conformational change of Bax; its binding epitope is not accessible in intact cells. As shown in Figure 6B, YTH-6A7-fluorescence intensity was increased in BL22-treated cells compared with medium controls, indicating that Bax underwent conformational changes in the presence of the immunotoxin. Because Bax cleavage and conformational changes have been associated with activation of the protein, our observations suggest a critical role for Bax in the initiation of apoptosis. In combination with reduced levels of antiapoptotic proteins, Mcl-1, and XIAP, this observation may, at least in part, explain the onset of apoptosis in immunotoxin-treated CLL cells.
Modulation of BL22 cytotoxicity
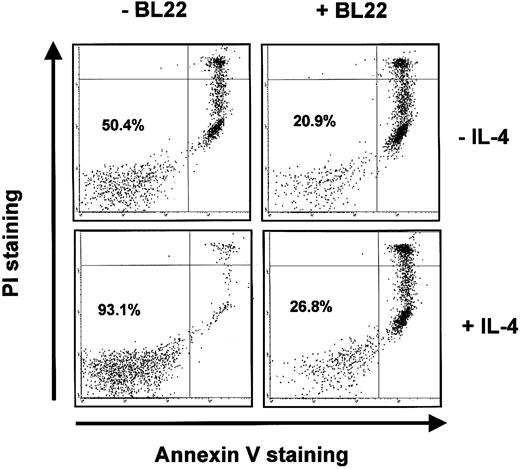
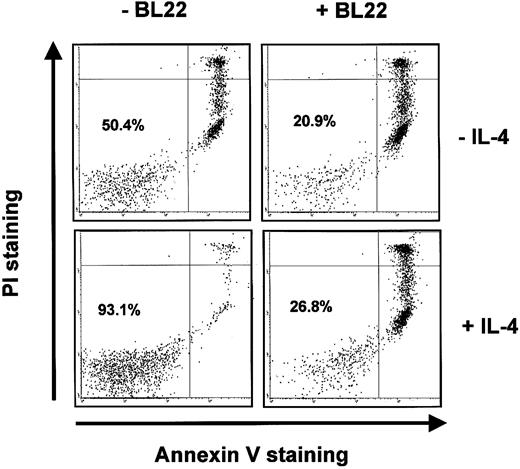
BL22 was also active in the presence of IL-4. IL-4 inhibited spontaneous apoptosis of B-CLL cells in culture. In the CLL sample shown in Figure 7, IL-4 increased the numbers of viable (ie, Annexin V-/PI-) cells from approximately 50% to 93%. Nevertheless, IL-4 failed to prevent BL22-induced apoptosis. Only 27% of CLL cells remained viable in a culture supplemented with BL22 and IL-4 (Figure 7).
Induction of apoptosis by BL22 in the presence of IL-4. B-CLL cells were grown for 72 hours in the presence or absence of 10 ng/mL IL-4 and 1000 ng/mL BL22. Cells were then stained with PI and Annexin V-FITC, as described in “Patients, materials, and methods.” Numbers indicate the percentages of viable cells (Annexin V-/PI-).
Induction of apoptosis by BL22 in the presence of IL-4. B-CLL cells were grown for 72 hours in the presence or absence of 10 ng/mL IL-4 and 1000 ng/mL BL22. Cells were then stained with PI and Annexin V-FITC, as described in “Patients, materials, and methods.” Numbers indicate the percentages of viable cells (Annexin V-/PI-).
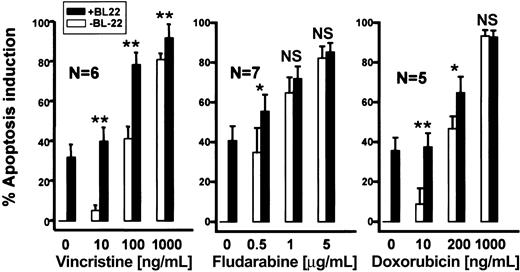
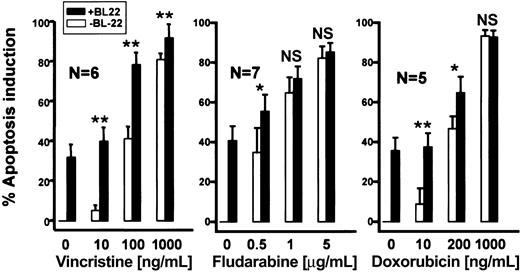
Next, we determined whether the cytotoxicity of BL22 was enhanced in combination with chemotherapeutic anticancer drugs. To this end, CLL cells were incubated for 72 hours with anticancer drugs. Figure 8 shows dose-response evaluations for fludarabine, vincristine, or doxorubicin in combination with BL22. Approximately 40% of CLL cells underwent apoptosis when exposed to 100 ng/mL vincristine. BL22 at 1000 ng/mL displayed similar cytotoxicity. The combination of both agents, however, displayed a marked antileukemic effect, killing almost 80% of CLL cells. The same percentage of apoptotic cells was induced by the 10-fold increase in concentration of single-agent vincristine. The antileukemic activity of fludarabine and doxorubicin was also augmented by BL22, albeit to a lesser extent (Figure 8). In summary, simultaneous exposure to the immunotoxin and low concentrations of anticancer drugs resulted in increased cytotoxicity.
Antileukemic activity of BL22 in combination with chemotherapeutic anticancer agents. B-CLL cells were cultured for 72 hours at indicated concentrations of anticancer drugs with or without 1000 ng/mL BL22. Apoptosis induction was assessed using Annexin V/PI staining, as described in “Patients, materials, and methods.” Numbers of analyzed patient samples are indicated. Results are presented as means ± SEM. Paired t test was performed for comparison of apoptosis induction by BL22 in the presence or absence of chemotherapeutic anticancer drugs. Significance levels are indicated: *P < .05; **P < .01; NS indicates not significant (P > .05).
Antileukemic activity of BL22 in combination with chemotherapeutic anticancer agents. B-CLL cells were cultured for 72 hours at indicated concentrations of anticancer drugs with or without 1000 ng/mL BL22. Apoptosis induction was assessed using Annexin V/PI staining, as described in “Patients, materials, and methods.” Numbers of analyzed patient samples are indicated. Results are presented as means ± SEM. Paired t test was performed for comparison of apoptosis induction by BL22 in the presence or absence of chemotherapeutic anticancer drugs. Significance levels are indicated: *P < .05; **P < .01; NS indicates not significant (P > .05).
Improved toxicity of a mutated immunotoxin
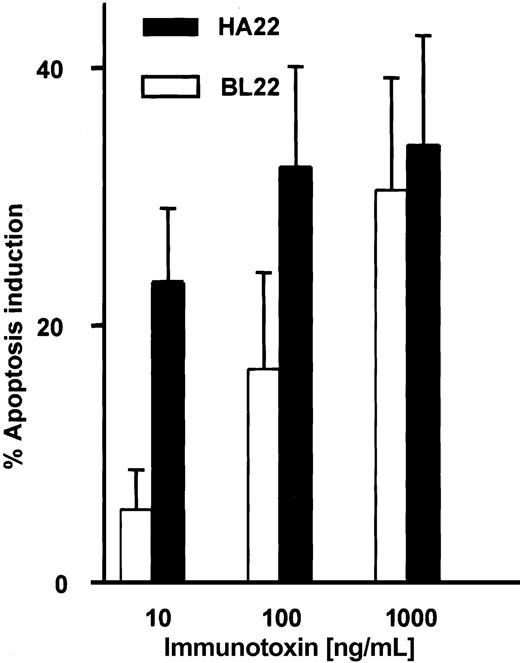
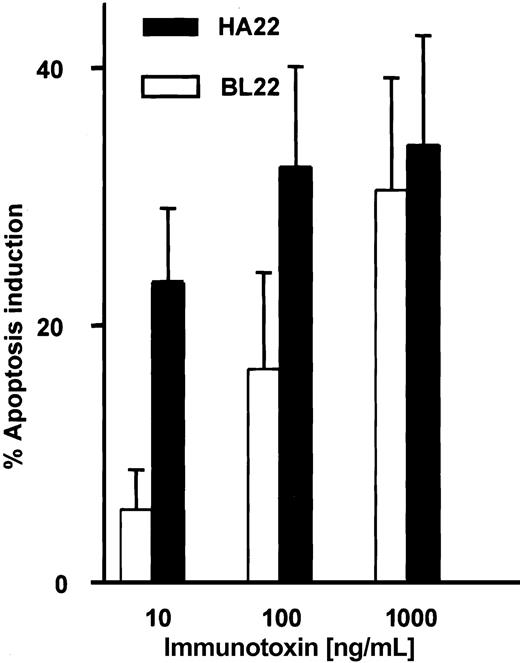
A mutated single-chain immunotoxin with increased affinity for CD22 has been developed from BL22 by mutating 2 amino acid residues in the complementarity-determining region of the heavy chain.31 HA22 is a newly developed disulfide-linked immunotoxin that contains these mutations in its binding region. We studied the activity of this immunotoxin in comparison with BL22. Induction of apoptosis by BL22 and the mutant immunotoxin was dose dependent at levels ranging from 10 to 1000 ng/mL (Figure 9). At the high concentration of 1000 ng/mL, both immunotoxins were equally cytotoxic. However, HA22 displayed much higher antileukemic activity than BL22 at 10 ng/mL and 100 ng/mL.
Cytotoxic activity of the mutated anti-CD22 immuntoxin HA22. B-CLL cells were cultured for 72 hours with indicated concentrations of BL22 or the mutated immunotoxin HA22. Apoptosis induction was determined by Annexin V staining, as described in the legend to Figure 2. Shown are means of apoptosis induction ± SEM from 7 patient samples.
Cytotoxic activity of the mutated anti-CD22 immuntoxin HA22. B-CLL cells were cultured for 72 hours with indicated concentrations of BL22 or the mutated immunotoxin HA22. Apoptosis induction was determined by Annexin V staining, as described in the legend to Figure 2. Shown are means of apoptosis induction ± SEM from 7 patient samples.
Discussion
In this study we investigated the effects of a recombinant anti-CD22 immunotoxin in B-CLL cells. We found that the immunotoxin exerts its cytotoxicity through programmed cell death caused by the activation of caspase-9, caspase-3, and PARP cleavage. The relevant role of caspases was confirmed by almost complete reversal of apoptosis in the presence of Z-VAD.fmk, a competitive and irreversible inhibitor of all caspases.35 Death of CLL cells by the immunotoxin involves alterations of the mitochondrial membrane, as demonstrated by the loss of membrane potential, Δψm. Mitochondria play a critical role in the intrinsic pathway of apoptosis. They release cytochrome c, which contributes to the activation of caspase-9, which, in turn, activates the executioner caspase-3.36,37
The observed predominance of caspase-dependent apoptosis for the death of CLL cells was unexpected. Several investigations have revealed defects in the induction of programmed cell death in CLL cells.5,6,38 Long-lived B-CLL cells are protected by Bcl-2 and Mcl-1 expression, though Bax and Bak may act as proapoptotic factors.5,39-41 In addition, the phosphatidylinositol-3 kinase pathway is involved in the defect of programmed cell death in B-CLL.10,42 Furthermore, caspase-independent pathways leading to apoptosis have been described in various cellular models, including CLL.43-45
Pseudomonas exotoxin and PE38-based immunotoxins can kill cells by means other than apoptosis, and previous studies suggest that immunotoxin-induced apoptosis plays a minor role in cytotoxicity compared with chemotherapeutic drugs.26,27 Pseudomonas exotoxin interferes with elongation factor-2, thereby inhibiting protein synthesis.24 However, protein synthesis inhibition does not always lead to apoptosis. For instance, inhibition of protein synthesis by cycloheximide can cause cell cycle arrest rather than cell death in certain cell models, or it can even block the induction of apoptosis by other stimuli.46-48
CLL cells and normal lymphocytes are sensitive to the induction of programmed cell death by cycloheximide.49 Data presented here show that antiapoptotic protein Mcl-1 levels are reduced in BL22-treated CLL cells. This would alter the balance of proapoptotic and antiapoptotic factors and would facilitate mitochondrial activation of programmed cell death. In addition, XIAP levels are reduced. XIAP is a member of the IAP (inhibitors of caspase) family of proteins, which inhibit the activity of executioner caspases.50
Furthermore, BL22 induces changes in the proapoptotic protein Bax. A cleaved Bax protein of 18 kDa was observed when CLL cells were exposed to BL22. The appearance of a 18-kDa Bax product has been reported previously in apoptotic B-CLL cells.45,51 The shorter molecule appears to be more potent in apoptosis induction than the 21-kDa Bax protein.52,53 We also noted changes in the conformation of Bax when treating CLL cells with BL22. Bax conformation is altered during translocation to the outer mitochondrial membrane, a pivotal step in the process of apoptosis initiation in CLL cells.52 Conformational changes of Bax have also been described in apoptotic CLL cells exposed to proteasome inhibitors.54 In conclusion, the decreased expression of antiapoptotic proteins and activation of the proapoptotic protein Bax are involved in apoptosis initiation. We have shown here that damage to mitochondria is an early event in immunotoxin-treated CLL cells that precedes the activation of caspase-3.
Damage to mitochondria initiates intrinsic pathways of apoptosis, associated with the activation of caspase-9. In fact, procaspase-9 levels are reduced in BL22-treated cells. We observed, however, a minor reduction of procaspase-8 levels after treatment with BL22. This could result from additional activation of extrinsic pathways of apoptosis, though expression of the CD95 (Fas) death receptor was not increased. Alternatively, caspase-8 could be activated as a downstream event, mediated by caspase-9 and caspase-3 without activation of the Fas death receptor.55 Regardless of the exact mechanism of caspase-8 activation, the contribution of this caspase appears to be less significant than that of caspase-9 in BL22-treated cells.
In accord with findings of previous reports, we observed that, though they are resistant to programmed cell death in vivo in peripheral blood, a portion of CLL cells die spontaneously in tissue culture.56 Changes in the microenvironment or deprivation of serum factors may be responsible for this finding. IL-4 has been identified as an antiapoptotic survival factor, increasing Bcl-2 protein levels for extended periods in cultured CLL cells.29,57 We found that the antiapoptotic signal produced by IL-4 abrogates the induction of spontaneous apoptosis in culture but cannot overcome the cytotoxicity of the immunotoxin.
BL22 is under clinical evaluation for the treatment of leukemias and malignant lymphomas. We were interested in combinations with anticancer drugs because the monoclonal anti-CD20 antibody rituximab has shown potentiated activity when administered with fludarabine.58,59 In addition, rituximab overcomes Bcl-2-associated resistance to vincristine- and anthracycline-based chemotherapy in patients with diffuse large-cell lymphomas.60 In our study BL22 augmented the antileukemic activity of anticancer drugs. The observed effect was most striking in combination with vincristine but was also detectable with fludarabine and doxorubicin. Augmentation of the cytotoxicity was most noticeable at low concentrations of anticancer drugs. Because the adverse effects of PE38-based immunotoxins are different from those of vincristine or fludarabine, it appears possible that such an approach might improve the treatment of CLL and even reduce severe adverse events. To our knowledge such chemo-immunotherapy with immunotoxins has not yet been investigated clinically. Further preclinical studies in tissue culture are, however, required to optimize the conditions of such a therapeutic strategy. Most protocols for chemotherapy administration lead to shorter plasma half-lives than the incubation periods in the present investigation.
A clinically important observation of our study was that flow cytometric quantitation of CD22 expression reliably predicted the sensitivity of B-CLL cells to BL22. In contrast to these findings, an anti-CD25 immunotoxin was less cytotoxic in some CLL samples than in hairy cell leukemia despite higher expression of the target antigen.28 In several previous studies, radiolabeled antibodies have been used to quantitate the numbers of binding sites.20,21 Most clinical centers have access to flow cytometry, whereas radioreceptor assays are not always feasible. Our finding may thus simplify clinical applications for BL22.
The low numbers of target molecules in approximately 50% of CLL samples may limit the application of BL22. We have previously reported that CLL cells can be sensitized to an anti-CD25 immunotoxin by up-regulation of the cellular target with oligodeoxynucleotides.30 This did not occur with the anti-CD22 immunotoxin.30 A different strategy to overcome the limitation of binding to target cells is illustrated by the development of the immunotoxin HA22. To design this immunotoxin, the binding region of antibody RFB4, from which BL22 had been derived, was analyzed for mutational hotspots.31 Phage display was used to identify mutations in the heavy chain complementarity-determining region 3 from antibody RFB4. The resultant immunotoxin disclosed higher binding affinity than BL22 toward CD22+ Daudi cells.31 Our current results show HA22 is more active against leukemic cells of CLL patients. This immunotoxin appears to be particularly useful for the treatment of CLL because it is highly cytotoxic at low dose levels that should be well tolerated in patients.
In conclusion, we have shown that Pseudomonas exotoxin-based immunotoxins overcome defects of apoptosis in CLL cells and kill through caspase-3-mediated programmed cell death. The cytotoxicity of the anti-CD22 immunotoxins is predicted by flow cytometric analysis. Introducing mutations into the binding region and combining treatment with chemotherapeutic anticancer agents offer new options to improve the antileukemic efficacy of immunotoxins against CLL cells.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1317.
Supported by Deutsche Forschungsgemeinschaft grant De771.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.