Abstract
Multiple myeloma is an incurable malignancy, and there is currently no mouse model that fully recapitulates the development and progression of the disease. We now describe a transgenic mouse that expresses a Bcl-XL transgene under the control of the 3′κ immunoglobulin light chain enhancer, which is most active in murine B cells in late developmental stages. These mice developed nonmalignant plasma cell foci in the bone marrow and soft tissues and hyaline tubular casts in the kidneys. Median survival of the 3′KE/Bcl-XL mice was similar to littermate controls. When the 3′KE/Bcl-XL mouse was crossed to an Eμ/c-Myc transgenic mouse, median survival of double transgenic progeny was 5.5 weeks. Peripheral blood and soft tissues were infiltrated with immature/mature B cells, and plasma cell lesions were identified in the bone marrow of all mice coexpressing Bcl-XL and c-Myc. These B- and plasma cell lesions demonstrated features consistent with malignancy. These results indicate that the 3′κ immunoglobulin light chain enhancer can effectively target expression of Bcl-XL to B cells in late developmental stages, and they provide direct evidence that Bcl-XL can contribute to plasmacytomagenesis. Furthermore, this murine model serves as an important step in developing a novel genetically induced mouse model of plasma cell malignancies exhibiting bone marrow involvement. (Blood. 2004;103:2779-2786)
Introduction
Multiple myeloma (MM) is an incurable expansion of malignant plasma cells within the bone marrow.1,2 Common complications of MM include bone lesions, anemia, susceptibility to infections, renal failure, and hypercalcemia.3 Standard treatments include steroids, DNA alkylating agents, and autologous peripheral blood stem cell transplantations, although they are not curative.2
Unlike other hematologic malignancies that often have common genetic abnormalities, there is no pathognomonic genetic lesion described for MM.4,5 Regardless, up-regulation of antiapoptotic proteins such as Bcl-XL and Mcl-1 has been identified in MM.6 Indeed, expression levels of these proteins are heightened in human and murine MM cell lines, especially upon stimulation with interleukin-6 (IL-6), an important growth factor for MM.7,8 While Bcl-XL and Mcl-1 share similar structure and function, recent studies have identified unique roles for these proteins in MM cells. Bcl-XL is shown to play a role in making MM cells resistant to chemotherapeutic drugs,9 although Mcl-1 is thought to be more influential in promoting tumor survival.10 These studies have been mostly performed in MM cell lines, however, and the role of elevated Bcl-XL or Mcl-1 expression in plasma cells and/or MM has not been tested in an animal system. Furthermore, the contribution of these proteins to plasmacytomagenesis has not yet been demonstrated.
Many different lymphoid malignancy transgenic animal models use the mu heavy chain immunoglobulin (Ig) enhancer (Eμ) to target transgene expression to B and/or T cells. Because we were interested in specifically targeting Bcl-XL expression to B cells in late developmental stages, we chose a more suitable enhancer for our model. We previously demonstrated that the κ Ig gene 3′ enhancer (3′KE) is most active in mature B and plasma cells,11 and we used it to direct transgenic Bcl-XL expression in mice. We also crossed the 3′KE/Bcl-XL mouse to an Eμ/c-Myc mouse to evaluate the cooperativity of Bcl-XL and c-Myc in promoting B- and plasma cell malignancy.
Materials and methods
Transgenic construct
The 3′κ enhancer and Vκ21 promoter (3′KE/KP) were isolated from pK3′E.KP.LUC.11 The Eμ and TK promoter were excised from a Bcl-XL construct12 and were replaced with the 3′KE/KP to form p3′KE/Bcl-XL. The transgenic vector was purified by CsCl centrifugation, linearized, and sent to the University of Minnesota Mouse Genetics Laboratory for microinjection.
Animal housing and husbandry
All mice were housed in a specific pathogen-free environment under Institutional Animal Care and Use Committee (IACUC) protocol no. 0006A56361. 3′KE/Bcl-XL mice were of the FVB/N strain, and Eμ/c-Myc mice (Jackson Laboratory, Bar Harbor, ME) were of the C57BL/6 strain. For experiments crossing the 3′KE/Bcl-XL to the Eμ/c-Myc mice, all comparisons were made among F1 progeny.
DNA purification
Genomic DNA from tail snips was obtained as previously described.13 Proteinase K (20 mg/mL) was added to the lysis solution, and the samples were incubated overnight at 55°C to ensure complete sample digestion.
Genotyping
A total of 100 ng genomic DNA was amplified by polymerase chain reaction (PCR). We used the following primer sets: Bcl-XL-FLAG, 5′-GACTACAAGGACGACGATGACAAG-3′; RBCLDOWN, 5′-AGTGGATGGTCAGTGTCTGGTCAC-3′; c-Myc-1MYC, 5′-CAGCTGGCGTAATAGCGAAGAG-3′; 2MYC, 5′-CTGTGACTGGTGAGTACTCAACC-3′; IL-2 (PCR amplification control)-1IL-2, 5′-CTAGGCCACAGAATTGAAAGATCT-3′; 2IL-2, 5′-GTAGGTGGAAATTCTAGCATCATCC-3′.
Southern blotting
To identify founders, 10 μg genomic DNA was digested with BglII (Invitrogen, Rockville, MD), and Bcl-XL cDNA was used as a probe. To determine clonality of isolated samples, 10 μg genomic DNA was digested with BamHI and was probed with DNA corresponding to the κ locus (Invitrogen). Southern blotting was performed as previously described.13
Reverse transcriptase (RT)-PCR
RNA samples were isolated using TRIzol and were treated with DNase I (Invitrogen). cDNA was synthesized using Superscript RT (Invitrogen). Template cDNA was amplified using the Bcl-XL primers used for genotyping. Actin primers were used as a control (ACTINUP, 5′-CCTAAGGCCAACCGTGAAAAG-3′; ACTINDOWN, 5′-TCTTCATGGTGCTAGGAGCCA-3′).
Western blotting
Western blot analysis was performed as previously described14 using the following antibodies: anti-Bcl-XL (Santa Cruz Biotechnology, Santa Cruz, CA), anti-FLAG (Sigma, St Louis, MO), antiactin (Santa Cruz Biotechnology), and antimouse Ig (Amersham, Cleveland, OH). To detect serum Ig proteins, 0.5 μL serum was loaded in each well. To detect urine Ig proteins, protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA), and 50 μg protein was loaded into each well.
Staining, flow cytometry, and fluorescence-activated cell sorting (FACS)
The following monoclonal antibodies (mAbs) were used for cell staining: anti-CD-3, anti-B220, anti-CD138, anti-IgM, anti-IgD, and anti-heat-stable antigen (HSA) (all antibodies from BD Pharmingen, San Diego, CA, except anti-HSA [T. Waldschmidt, University of Iowa]). Standard flow cytometric analysis was carried out on a FACSCalibur machine (BD Pharmingen) at the University of Minnesota Cancer Center Flow Cytometry Core, and data were collected and analyzed using Cellquest Pro (BD Pharmingen). Stained cells were sorted on a FACSVantage machine (BD Pharmingen).
Cell culture
Cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Invitrogen).
Cell survival
Cells were stained with 0.4% trypan blue (Sigma) and counted on a hemacytometer (Fisher Scientific, Pittsburgh, PA).
ELISA
Enzyme-linked immunosorbent assays (ELISAs) were performed by the University of Minnesota Cytokine Reference Laboratory as previously described.15
Histology and IHC
Mice were humanely killed and necropsied, and each opened mouse carcass, including internal organs, was fixed in 10% formalin (Sigma). Selected tissues were embedded in paraffin and sectioned at 3 to 5 μm. Tissues were either stained with hematoxylin and eosin (H&E) or prepared for immunohistochemistry (IHC). For IHC, tissues were steamed for 30 minutes in 1 mM EDTA (ethylenediaminetetraacetic acid), pH 8.0, and blocked with 3% hydrogen peroxide, Avidin/Biotin Block (Vector, Burlingame, CA), and Dako Protein Block (Dako, Carpinteria, CA). Tissues were incubated overnight with biotinylated anti-B220 or anti-CD138 antibodies (BD Pharmingen) at 4°C. Tissues were incubated with streptavidin horseradish peroxidase (HRP) enzyme conjugate (Dako) for 15 minutes, and Vector Nova Red Substrate (Vector) was used for color determination. Sections were counterstained with Mayer hematoxylin.
Results
Generation of 3′KE/Bcl-XL transgenic mice
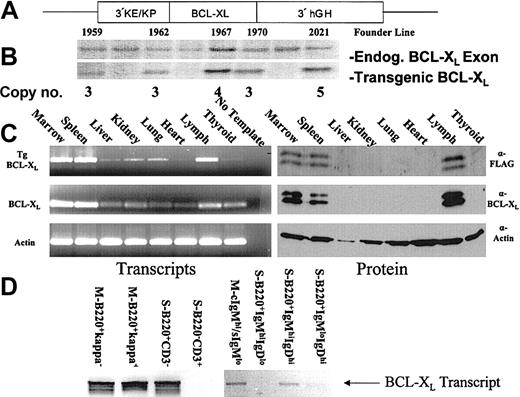
To examine the developmental activity of the 3′KE and the effect of dysregulation of apoptosis in B lymphocytes, we constructed the plasmid vector p3′KE/Bcl-XL in which the 3′KE and kappa Vκ21 promoter (KP) are paired with murine Bcl-XL cDNA containing the FLAG epitope (Figure 1A). The FLAG epitope was placed at the 5′ end of the Bcl-XL cDNA as previously described.12 The human growth hormone (hGH) minigene sequence16 was placed at the 3′ end of the construct to provide RNA splicing and processing. Linearized p3′KE/Bcl-XL was injected into male pronuclei of FVB/N mouse embryos. Southern blotting identified 5 transgenic founders, and transgene copy number ranged from 3 to 5 copies (Figure 1B). Of the 5 founders, 4 demonstrated germ-line transmission of the transgene, and the founder lines 1962, 1967, and 2021 were maintained and characterized. All 3 founder lines demonstrated abnormal B-lymphocyte biology. Data presented in this report were mainly collected using animals from the 1967 founder line, with a few samples from the 2021 founder line. We have noted the founder lineage and age of each mouse for each experiment.
Construction and expression analysis of the Bcl-XL transgenic mouse. (A) Diagram of the 3′KE/Bcl-XL transgenic construct. (B) Southern blot analysis of tail-snip DNA to identify founders. (C) RT-PCR and Western blot analysis to determine tissue-specific transgene expression. (D) RT-PCR to detect transcripts in FACS-sorted lymphocytes (S indicates spleen; M, marrow).
Construction and expression analysis of the Bcl-XL transgenic mouse. (A) Diagram of the 3′KE/Bcl-XL transgenic construct. (B) Southern blot analysis of tail-snip DNA to identify founders. (C) RT-PCR and Western blot analysis to determine tissue-specific transgene expression. (D) RT-PCR to detect transcripts in FACS-sorted lymphocytes (S indicates spleen; M, marrow).
Transgene expression is tissue and B-cell specific
To characterize transgene expression, RNA and protein were isolated from the marrow, spleen, liver, kidney, lung, heart, lymph, and thyroid of a 6-week-old 3′KE/Bcl-XL mouse from the 1967 founder line. RT-PCR was used to detect transcripts in the tissue samples. The transgenic Bcl-XL was tagged with the FLAG epitope, and both endogenous and transgenic Bcl-XL levels were determined. Transgenic Bcl-XL levels were highest in the marrow, spleen, and lymph nodes (Figure 1C). Low levels of transcripts detected in other tissues were likely due to transgene-expressing lymphocytes in peripheral circulation that migrated to those locations, including microscopic foci of lymphocytes found upon histopathological examination. Transgenic Bcl-XL expression at the protein level was confined to the marrow, spleen, and lymph (Figure 1C). Two isoforms of Bcl-XL are seen—Bcl-XL and its alternatively spliced Bcl-X ΔTM (loss of transmembrane domain).17
Next we used FACS to sort different cell populations of lymphocytes and RT-PCR to identify which cell populations harbored transgenic Bcl-XL transcripts. All tissue samples came from 6-week-old 3′KE/Bcl-XL mice from the 1967 founder line. Marrow cells from 3′KE/Bcl-XL mice were stained with anti-κ, anti-B220, anti-IgM-fluorescein isothiocyanate (FITC) (surface), and/or anti-IgM-phycoerythrin (PE) (cytoplasmic) antibodies. Spleen cells from 3′KE/Bcl-XL mice were stained with anti-B220, anti-CD3, anti-IgM, and/or anti-IgD antibodies. Transgenic transcripts were detected in the pan-B-cell (B220+/κ-), immature/mature B-cell (B220+/κ+), and intracellular IgM-staining plasma cell (cytoplasmic IgMhi/surface IgMlo [cIgMhi/sIgMlo]) populations of the bone marrow (Figure 1D). Transgenic transcripts were detected in the pan-B-cell (B220+/CD3-) and immature/transitional B-cell (IgMhiIgDhiB220+) spleen populations but not detected in the pan-T-cell (B220-/CD3+), immature B (IgMhiIgDloB220+), or mature B (IgMloIgDhiB220+) spleen populations (Figure 1D). This analysis demonstrates that 3′KE activity and transgene expression is restricted to B cells but not T cells. Furthermore, transcripts can be detected in immature/transitional B cells of the spleen and IgM-expressing bone marrow plasma cells, correlating with the abnormal lymphocyte populations detected by flow cytometry and histopathological analysis in this report.
3′KE/Bcl-XL mice have altered lymphocyte populations
Because a B-cell-restricted transcriptional enhancer was used to drive Bcl-XL expression in a transgenic mouse, we expected the 3′KE/Bcl-XL mice to have altered lymphocyte compartments. Samples of peripheral blood from 12-week-old 3′KE/Bcl-XL mice (n = 3) from the 1967 founder line and littermate controls (LMCs) (n = 2) were analyzed on a Hemavet automated cell counter (CDC Technologies, Oxford, CT). All 3′KE/Bcl-XL animals demonstrated a lymphocytosis compared with LMCs, with an average count of 13.2 × 103 ± 2.26 × 103 lymphocytes per microliter versus 3.5 × 103 ± 0.21 × 103 lymphocytes per microliter (P = .02).
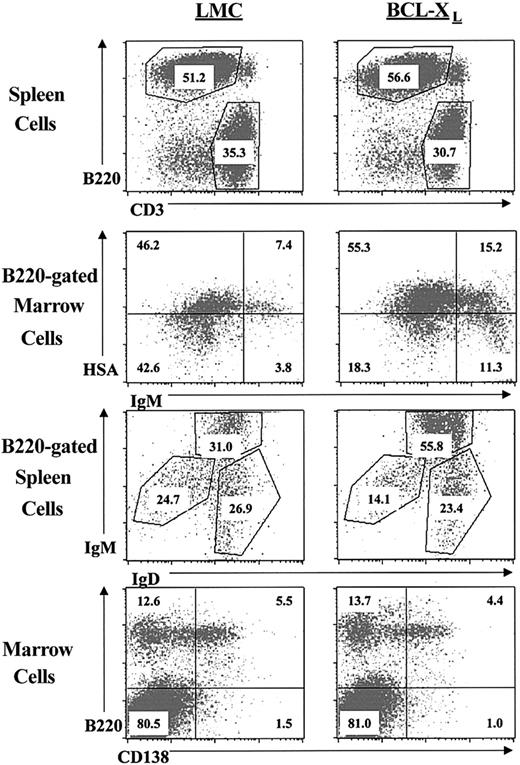
To further characterize the differences in the lymphocyte populations, samples of cells from 12-week-old LMC or 3′KE/Bcl-XL mice from the 1967 founder line were stained with fluorescently labeled antibodies corresponding to different B- and T-lymphocyte cell surface markers and analyzed by flow cytometry. Splenocytes from age-matched LMC and 3′KE/Bcl-XL mice were stained with anti-B220 and anti-CD3 antibodies. The percentages of B and T lymphocytes are similar in both groups (Figure 2 and Table 1).
Immunophenotypic analysis of lymphocytes in the transgenic and littermate control mice.
Immunophenotypic analysis of lymphocytes in the transgenic and littermate control mice.
Given that the 3′KE is not strongly active until the immature and mature stages of B-cell development, we did not expect to see altered lymphocyte populations until those stages and beyond. Indeed, we did not find any differences in the BP-1+ pre-B-cell populations between the 3′KE/Bcl-XL and LMC mice (data not shown). Marrow cells from 3′KE/Bcl-XL transgenic and LMC mice were stained with anti-B220, anti-IgM, and anti-HSA antibodies, and spleen cells from 3′KE/Bcl-XL transgenic and LMC mice were stained with anti-B220, anti-IgD, and anti-IgM antibodies. The 3′KE/Bcl-XL transgenic animals showed a significant increase in the B220+/IgMhi/IgDhi immature/transitional B cells of the spleen as well as the B220+/IgM+ immature (HSAhi) and mature (HSAlo) B cells of the marrow (Figure 2 and Table 1). These expanded populations correlate to the transgenic transcriptional analysis in Figure 1. Marrow cells were also stained with anti-B220 and anti-CD138 antibodies to examine the plasma cell compartments of LMC and 3′KE/Bcl-XL transgenic mice. No significant differences were seen among the CD138hiB220lo plasma cell populations of the 2 groups (Figure 2 and Table 1).
3′KE/BCL-XL mice have elevated serum immunoglobulin levels
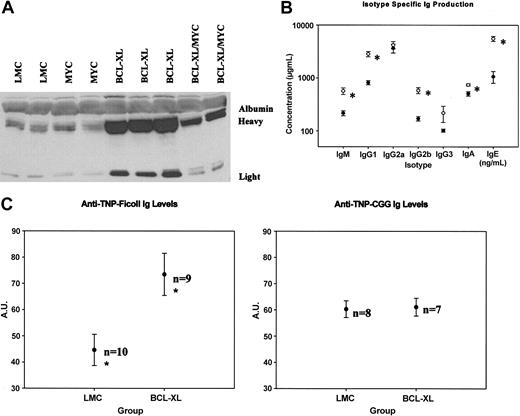
We collected serum samples from 12-week-old 1967 founder line 3′KE/Bcl-XL and LMC mice. Western blotting of serum samples using an antimouse Ig antibody demonstrates that total amounts of heavy and light chain Ig proteins were significantly elevated in the 3′KE/Bcl-XL mice compared with LMCs (Figure 3A). We then used ELISAs to determine isotype-specific serum Ig levels. 3′KE/Bcl-XL animals demonstrated significantly (P < .05) elevated levels of the IgM, IgG1, IgG2b, IgA, and IgE isotypes compared with LMCs (Figure 3B). Serum samples were analyzed by conventional serum protein electrophoresis as well as 2-dimensional gel electrophoresis with subsequent Western blotting to detect Ig proteins, and although the γ-globulin fractions in the 3′KE/Bcl-XL mice were expanded, no clonal spikes were observed in any of the samples (data not shown).
Serum analysis of the transgenic mice. (A) Western blot analysis of serum from representative mice. (B) ELISAs of serum to measure concentration of individual immunoglobulin isotypes in 3′KE/Bcl-XL (○; n = 12) and LMC (•; n = 9) mice. (C) TNP-Ficoll- and TNP-CGG-specific immunoglobulin levels in 3′KE/Bcl-XL and LMC mice. A.U. indicates arbitrary units. Error bars indicate ± SEM. *P < .05.
Serum analysis of the transgenic mice. (A) Western blot analysis of serum from representative mice. (B) ELISAs of serum to measure concentration of individual immunoglobulin isotypes in 3′KE/Bcl-XL (○; n = 12) and LMC (•; n = 9) mice. (C) TNP-Ficoll- and TNP-CGG-specific immunoglobulin levels in 3′KE/Bcl-XL and LMC mice. A.U. indicates arbitrary units. Error bars indicate ± SEM. *P < .05.
3′KE/Bcl-XL mice make more T-cell-independent antigen-specific immunoglobulins than LMCs
Given the putative apoptosis-resistant B lymphocytes and elevated serum Ig levels in the 3′KE/Bcl-XL animals, we wanted to test whether the 3′KE/Bcl-XL animals respond to antigenic challenge and whether they make more antigen-specific Igs than LMCs. We rationalized that such an antigenic challenge would help us to distinguish between a lymphoproliferative disease and a malignancy, because malignant cells would unlikely be responsive to antigen. Eight-week-old 1967 founder line 3′KE/Bcl-XL and LMC animals were immunized with trinitrophenyl (TNP)-Ficoll, a T-cell-independent antigen, or trinitrophenyl (TNP)-chicken γ-globulin (CGG), a T-cell-dependent antigen. Primary immunizations were given in Freund complete adjuvant (FCA), and secondary immunizations were given 14 days later in Freund incomplete adjuvant (FIA). Serum samples were collected 14 days after the secondary immunization, a time point when maximum antibody production was expected. ELISAs were performed to estimate antigen-specific Ig levels. 3′KE/Bcl-XL mice make 65% more TNP-Ficoll-specific Igs than LMCs (P < .05), although 3′KE/Bcl-XL and LMC animals make similar amounts of TNP-CGG-specific Igs (Figure 3C).
3′KE/Bcl-XL mice develop plasma cell foci and renal tubular casts
Necropsies of 3′KE/Bcl-XL and LMC mice were performed, and histologic sections of the kidneys, spleen, lymph nodes, liver, lung, heart, gastrointestinal (GI) tract, and long and flat bones were made and stained with hematoxylin and eosin (H&E). No abnormalities were detected in the LMC mice studied. Analyses of the 3′KE/Bcl-XL mice revealed distinct histopathologic lesions in the 3 founder lines studied, and the incidence and magnitude of microscopic lesions correlated directly with age of the animal. Histopathologic changes were detected in all 3′KE/Bcl-XL mice studied, ranging between 3 and 24 months of age.
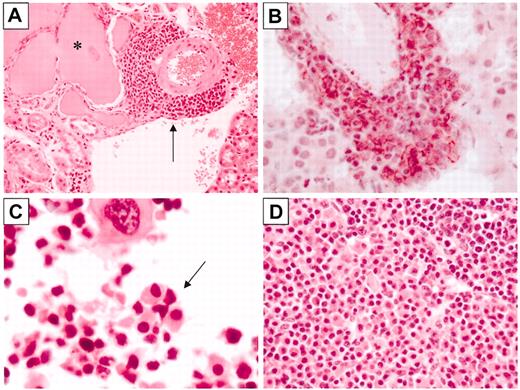
A number of 3′KE/Bcl-XL mice developed multiple perivascular foci of lymphocytes in the kidney, including a 23-month-old animal from the 2021 founder line (Figure 4A). Mice that had urine Ig levels detectable by Western blot analysis demonstrated the most pronounced pathology. Sections were immunostained with anti-CD138 or anti-B220 antibodies. A focus in a 9-month-old 1967 founder line 3′KE/Bcl-XL mouse immunostained intensely for surface CD138 but did not stain for surface B220 (data not shown), consistent with the cell surface phenotype of plasma cells (Figure 4B). Given the foci of plasma cells in the kidney and other tissues and the serum immunoglobulinemia, the total amount of Ig protein filtered by the kidney was increased in the transgenic animals. As a result, significant hyaline renal tubular casts, presumably Ig protein deposits, were detected in a number of animals. The 23-month-old animal depicted in Figure 4A demonstrated prominent hyaline casts affecting many renal tubules. Younger animals demonstrated less pronounced changes, with only 1 or 2 casts per kidney section.
Bcl-XL mice develop plasma cell pathology. (A) H&E-stained section of a kidney from a Bcl-XL mouse with a hyaline tubular cast (asterisk) and a perivascular focus of plasma cells (arrow). (B) CD138-immunostained (red reaction product) kidney section from a 3′KE/Bcl-XL mouse, counterstained with hematoxylin. (C) H&E-stained section of a femur of a 3′KE/Bcl-XL mouse demonstrating a bone marrow plasma cell focus (arrow). (D) H&E-stained section of a lymph node of a 3′KE/Bcl-XL mouse with plasma cell aggregates. Original magnifications: × 40 (A,D) and × 100 (B-C).
Bcl-XL mice develop plasma cell pathology. (A) H&E-stained section of a kidney from a Bcl-XL mouse with a hyaline tubular cast (asterisk) and a perivascular focus of plasma cells (arrow). (B) CD138-immunostained (red reaction product) kidney section from a 3′KE/Bcl-XL mouse, counterstained with hematoxylin. (C) H&E-stained section of a femur of a 3′KE/Bcl-XL mouse demonstrating a bone marrow plasma cell focus (arrow). (D) H&E-stained section of a lymph node of a 3′KE/Bcl-XL mouse with plasma cell aggregates. Original magnifications: × 40 (A,D) and × 100 (B-C).
Histopathological changes were also noted in the bone marrow and other lymphoid organs of the 3′KE/Bcl-XL mice. The percentage of lymphocytes present in the marrow cavity based on light microscopic evaluation was significantly increased compared with LMCs (data not shown).
Additionally, nests of plasma cells were found in the marrow of aged mice, including the 23-month-old 2021 founder line 3′KE/Bcl-XL mouse depicted in Figure 4C. Radiographs of multiple 3′KE/Bcl-XL and LMC mice were radiographically normal, with no evidence of diffuse demineralization or focal lesions in either group (data not shown). Some of the 3′KE/Bcl-XL mice contained sheets of plasma cells within lymph nodes, including the 9-month-old 1967 founder line mouse depicted in Figure 4D, and foci of CD138+ plasma cells and B220+ lymphocytes were detected in other nonlymphoid soft tissues, including the liver and lung (data not shown).
Bcl-XL and c-Myc cooperate to induce B-cell malignancies
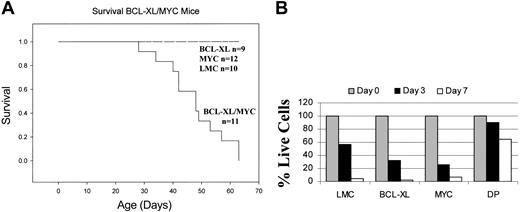
Dysregulation of the c-Myc protooncogene has been implicated in both human and murine plasmayctomas.4,18,19 As such, we crossed the 1967 founder line 3′KE/Bcl-XL mouse to the Eμ/c-Myc mouse to evaluate the cooperativity of Bcl-XL and c-Myc under the control of the 3′KE and the Eμ, respectively. Because the 3′KE/Bcl-XL mice are of the FVB/N strain and the Eμ/c-Myc mice are of the C57BL/6 strain, all comparisons were made among the syngeneic F1 progeny. The coexpression of antiapoptotic Bcl-XL and oncogenic c-Myc proved highly fatal, and 50% of the double transgenic mice died of a lymphoproliferative disorder at 5.5 weeks (Figure 5A). Mononuclear spleen cells from 4-week-old mice from all 4 genotypes constituting the F1 progeny were cultured in RPMI media, and the percentage of viable cells was estimated by trypan blue staining at days 3 and 7. Most cells from the LMC and single transgenic mice were dead at day 7, while more than 60% of the double transgenic cells were viable (Figure 5B). Furthermore, more than 60% of splenocytes from the double transgenic mice were still viable after 4 weeks in culture (data not shown).
Bcl-XL and c-Myc cooperate to form fatal B-cell tumors. (A) Survival curves of double transgenic, single transgenic, and littermate control mice. (B) Survival of splenocytes from double transgenic, single transgenic, and littermate control mice in culture.
Bcl-XL and c-Myc cooperate to form fatal B-cell tumors. (A) Survival curves of double transgenic, single transgenic, and littermate control mice. (B) Survival of splenocytes from double transgenic, single transgenic, and littermate control mice in culture.
Samples of peripheral blood from 4-week-old double transgenic mice (n = 2) and LMCs (n = 2) were analyzed on a Hemavet automated cell counter. All transgenic animals demonstrated a severe lymphocytosis compared with LMCs, with an average count of 79.16 × 103 ± 3.08 × 103 lymphocytes per microliter versus 6.32 × 103 ± 2.74 × 103 lymphocytes per microliter (P = .02). Peripheral blood mononuclear cells were stained with anti-κ and anti-B220 antibodies; 52.78% ± 6.23% of the peripheral blood mononuclear cells of the double transgenic mice were B220+/κ+, reflecting immature/mature B-cell populations, versus 5.38% ± 0.24% of the LMC mice cells. Serum Ig levels were detected by Western blotting, and while there were more serum heavy and light chain Ig proteins in the double transgenic mice than in the LMC or c-Myc mice, the Bcl-XL mice had the most elevated levels (Figure 3A).
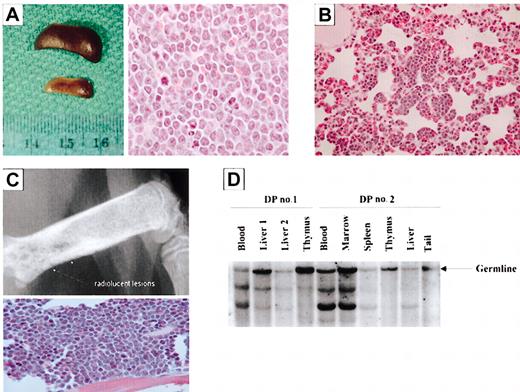
Necropsies were performed on animals exhibiting excessive morbidity between 4 and 6 weeks of age, and all double transgenic animals demonstrated a very severe splenomegaly (Figure 6A). In the liver, multiple beige nodules, up to 2 mm in diameter, were seen in all transgenic animals. Histologic sections of the kidneys, spleen, lymph nodes, liver, lung, heart, GI tract, and long and flat bones were made and stained with H&E. A multifocal to coalescent infiltration of mononuclear cells with large, round to polygonal hypochromatic nuclei and a low mitotic rate was seen in the kidneys, spleen, lymph node, liver, lung (Figure 6B), heart, thymus, and pancreas. Similar mononuclear cells were present in the sternal bone marrow. Radiographs of the double transgenic mice showed osteolytic lesions in the long bones (Figure 6C). When the corresponding areas of bone were sectioned and stained, large sheets of pleomorphic plasmacytic cells were found adjacent to lysed osseous trabeculae, sometimes penetrating the corticalis and infiltrating the surrounding soft tissue (Figure 6C). Southern blot analysis was used to examine the clonality of samples isolated from 2 double transgenic mice. Clonally related populations were detected in the blood, marrow, spleen, and liver nodule samples collected (Figure 6D). The invasive nature of the bone marrow plasma cell lesions and the clonally related B-cell populations demonstrated in the double transgenic mice illustrate features consistent with a malignant phenotype.
Mice coexpressing Bcl-XL and c-Myc develop clonal B- and plasma cell tumors. (A) (Left) Spleens from a double transgenic (top) and littermate control (bottom) mousee. (Right) H&E-stained spleen section of a double transgenic mouse. Cells are pleomorphic with an elevated mitotic index. (B) H&E-stained section of a lung of a double transgenic mouse. Aggregates of lymphocytes line the alveolar walls. (C) Radiography (top) demonstrates areas of bone lysis, and the H&E-stained section of the femur of a double transgenic mouse (bottom) shows replacement of bone marrow by sheets of plasma cells. (D) Southern analysis of samples collected from double transgenic mice (DP indicates double positive). Original magnifications: × 10 (C, top panel); × 40 (B; C, bottom panel); and × 100 (A, right panel).
Mice coexpressing Bcl-XL and c-Myc develop clonal B- and plasma cell tumors. (A) (Left) Spleens from a double transgenic (top) and littermate control (bottom) mousee. (Right) H&E-stained spleen section of a double transgenic mouse. Cells are pleomorphic with an elevated mitotic index. (B) H&E-stained section of a lung of a double transgenic mouse. Aggregates of lymphocytes line the alveolar walls. (C) Radiography (top) demonstrates areas of bone lysis, and the H&E-stained section of the femur of a double transgenic mouse (bottom) shows replacement of bone marrow by sheets of plasma cells. (D) Southern analysis of samples collected from double transgenic mice (DP indicates double positive). Original magnifications: × 10 (C, top panel); × 40 (B; C, bottom panel); and × 100 (A, right panel).
Discussion
This report describes the development of a novel transgenic system designed to direct Bcl-XL expression to B cells in late developmental stages, including plasma cells. The 3′KE/Bcl-XL mice have altered lymphocyte populations and serum immoglobulinemia, and nonmalignant bone marrow and extramedullary plasma cell foci and renal tubular casts develop in adult mice. When Bcl-XL and c-Myc are coexpressed under the control of the 3′κ and Eμ enhancers, respectively, highly fatal B-cell lesions develop. The predominant expanded lymphocytic population is composed of immature/mature B cells, and these cells infiltrate nearly every organ system studied. Notably, plasma cell lesions develop in the bone marrow, intimately interacting with the microenvironment to induce bone resorption. To our knowledge, this is the first report demonstrating genetically induced bone marrow plasma cell lesions in the mouse.
Bcl-XL is overexpressed in a variety of human cancers, including acute myelogenous leukemia,20 Hodgkin lymphoma,21 non-Hodgkin lymphoma,22 and HIV-associated lymphoma.23 Elevated Bcl-XL expression has also been demonstrated in human9 and murine7 myeloma cells. Bcl-XL's role in conferring increased survival and chemotherapeutic resistance to myeloma cells has been debated, however. IL-6, an important cytokine in plasma and myeloma cell biology, induces expression of Bcl-XL and Mcl-1, both antiapoptotic. Recent studies using antisense technology demonstrate that Mcl-1 rather than Bcl-XL is more influential in survival of human myeloma cell lines.10 A limitation of these studies, however, is that myeloma cell lines may not accurately reflect in vivo plasma cell biology. Our in vivo analysis of Bcl-XL demonstrates that elevated Bcl-XL expression alone or coexpressed with c-Myc can contribute to abnormal B-lymphocyte and plasma cell biology, because plasma cell foci or plasma cell lesions develop in the 3′KE/Bcl-XL or 3′KE/Bcl-XL × Eμ/c-Myc mice, respectively.
Notably, the 3′KE/Bcl-XL mice do not show an increase in the percentage of plasma cells constituting the bone marrow based on data collected by flow cytometry. We have demonstrated that transgenic Bcl-XL transcripts are present in IgM plasma cells and that 3′KE/Bcl-XL mice make more immunoglobulins than littermate controls. It is possible that transgenic Bcl-XL expression in plasma cells changes their microenvironment requirements, and these plasma cells migrate to atypical locations and form foci as detected upon necropsy. Indeed, recent work has demonstrated that enforced Bcl-XL expression can complement necessary B-cell survival and development of signaling pathways such as the BAFF-R (B-cell activating factor of the tumor necrosis factor family-receptor),24 and it is likely that Bcl-XL expression has altered B- and plasma cell survival and distribution. Thus, even though 12-week-old 3′KE/Bcl-XL mice have similar percentages and absolute numbers of plasma cells constituting their bone marrow compared with LMCs, they likely have a greater total number of plasma cells in extramedullary sites.
While the extramedullary plasma cell foci identified likely indicate that enforced Bcl-XL expression leads to increased numbers of plasma cells with an abnormal distribution, transgenic Bcl-XL expression alone may not confer bone marrow microenvironment independence. Bone marrow plasma cell foci were observed in 3′KE/Bcl-XL mice older than 1 year of age. Additionally, plasma cell tumors were only found in the bone marrow of our 3′KE/Bcl-XL × Eμ/c-Myc double transgenic mice. It is possible that the extramedullary plasma cell foci found in the 3′KE/Bcl-XL mice are a result of additional stochastic mutations that decrease reliance on the bone marrow microenvironment. Elevated Bcl-XL expression increases the likelihood that cells that acquire mutations survive. Because the 3′KE/Bcl-XL mice have a normal life span, there is sufficient time for cells to gain additional stochastic mutations. It is possible that these cells are able to migrate to extramedullary sites. The 3′KE/Bcl-XL × Eμ/c-Myc transgenic mice have very shortened life spans, however. Thus, there is a narrower window of opportunity for Bcl-XL/c-Myc double transgenic plasma cells to gain additional mutations compared with Bcl-XL single transgenic plasma cells, and a lack of additional mutations may confine the plasma cell lesions to the bone marrow where growth factors such as IL-6 are present in high concentrations.
Previous Bcl-XL transgenic mice have been described. One mouse uses the Eμ and a herpes TK promoter to drive Bcl-XL expression.12 This transgenic cassette drives high-level expression of Bcl-XL early in B-cell development, with a much lower level of expression in peripheral B cells.12 Another mouse uses the Eμ and an SV40 promoter to drive Bcl-XL, and Bcl-XL expression is higher in peripheral B cells than in bone marrow B cells.12 When either of the Eμ/Bcl-XL mice was crossed to an Eμ/c-Myc mouse, however, double transgenic mice developed highly fatal acute early B-cell leukemia, and the immunophenotypes of the malignant cells were essentially identical (T. Behrens, unpublished data, 1997). The Bcl-XL transgene used in our studies is driven by a combination of the 3′κ enhancer and Vκ21 promoter, uniquely targeting B cells in late developmental stages. These regulatory elements were likely influential in governing the phenotype of the malignant cells in our 3′KE/Bcl-XL × Eμ/c-Myc mice.
Potter et al have described the pristane oil-induced mouse plasmacytoma (MPC) model.25-27 The BALB/c strain of mice develops MPCs when given intraperitoneal injections of pristane oil. While this model has proved useful in understanding plasma cell transformation, MPCs only develop in extramedullary sites and are dependent upon the MPC-prone genetic background of the BALB/c mouse. Our transgenic model uses a targeted system to genetically induce plasma cell tumors that exhibit bone marrow involvement in an MPC-resistant strain of mice and demonstrates the cooperativity of Bcl-XL and c-Myc in B-cell tumor formation.
Our data indicate that the 3′KE can effectively target transgene expression of Bcl-XL to B cells in late developmental stages, including plasma cells. This targeted system serves as a model for directed development of plasma cell tumors exhibiting bone marrow involvement. Furthermore, these data provide direct evidence that Bcl-XL can contribute to plasmacytomagenesis, identifying the antiapoptotic protein as a potential molecular target for treating plasma cell tumors, including MM.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-10-3399.
Supported by the Cancer Biology Training Grant NCI T32 CA09138-27 and the Lung Science Training Grant NHLBI T32 HL07741-09 (M.L.), a University of Minnesota Academic Health Center Seed Grant, and a grant from the Leukemia Research Fund.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge the Cytokine Reference Laboratory (Angela Panoskaltsis-Mortari), Mouse Genetics Laboratory (Sandi Horn), Flow Cytometry Core (Julie Pribyl and Greg Veltri), Histopathology Core, Tom Waldschmidt (University of Iowa, antibodies), Tim Behrens (Bcl-XL cDNA), and Xiangdong Liu (cloning of p3′KE/Bcl-XL).