Abstract
The mechanisms governing migration and extramedullary dissemination of leukemic cells remain obscure. In this study the migration and in vivo homing to the bone marrow of nonobese diabetic severe combined immunodeficient (NOD/SCID) mice injected with human precursor-B acute lymphoblastic leukemia (ALL) cells in comparison to normal CD34+ progenitors (both cord blood and mobilized peripheral blood) was investigated. Although migration and homing of both cell populations was dependent on stromal cell-derived factor 1 (SDF-1)/CXCR4 interactions, major differences in receptor expression as well as the migratory capacity toward various concentrations of SDF-1 were found. Furthermore, unlike normal CD34+ progenitors, in vivo homing of the leukemic cells was superior when recipient NOD/SCID mice were not irradiated prior to transplantation. In addition, we report differences in the adhesion molecules activated following SDF-1 stimulation, documenting a major role for very late antigen 4 (VLA-4), but not VLA-5 and lymphocyte function-associated antigen-1 (LFA-1), in homing of precursor-B ALL cells. Interestingly, Toxin-B and pertussis toxin inhibited the homing of the leukemic cells but not that of normal CD34+ progenitors or normal CD10+/CD19+ precursor-B cells, revealing differences in CXCR4 signaling pathways that are based on changes that acquired by the leukemic cells. Altogether, our data provide new insights into different SDF-1–induced signaling, activation, and consequent motility between normal CD34+ and precursor-B ALL progenitors, which may lead to improved clinical protocols. (Blood. 2004;103: 2900-2907)
Introduction
Among the various leukemias, B-cell precursor acute lymphoblastic leukemia (ALL) represents the most common childhood leukemia. Approximately 70% of children are cured1 ; however, the need exists to improve the outcome in nonresponders, high-risk patients, and patients who relapse. Novel treatment strategies based on a better understanding of the biology of this type of leukemia, in particular the mechanisms that regulate migration and dissemination of the malignant clone, may assist in achieving this goal.
Immune deficient severe combined immunodeficient (SCID) and nonobese diabetic (NOD)/SCID mice have been used as a functional, preclinical model for in vivo engraftment and dissemination of human pre-B ALL cells.2,3 By using this model it was shown that the engraftment kinetics of human pre-B ALL blasts correlates with the prognosis of the disease in the original patients.4-6
Stromal-derived factor-1 (SDF-1; also named CXCL12), the ligand of the CXCR4 receptor, is constitutively produced by many cell types, including immature osteoblasts and endothelial cells within the bone marrow (BM) as well as by epithelial cells in many organs, including the central nervous system.7-9 Human and murine SDF-1 differ in only one amino acid and are cross-reactive.10 SDF-1 is the most powerful chemoattractant for undifferentiated human CD34+ hematopoietic progenitors11,12 and is the only chemokine known to induce high levels of directional migration of both human CD34+/CD38- and murine Sca-1+/ckit+/Lin- stem cells.13,14 We have previously shown the essential role of SDF-1/CXCR4 interactions in both homing to the murine BM and high-level multilineage repopulation by human CD34+/CD38-/low SCID repopulating cells (SRCs) in NOD/SCID mice that received transplants.8,13,15
SDF-1, originally cloned from a stromal cell line as a pre-B cell growth factor, is essential for normal B-cell development. Mice that lack SDF-1 or CXCR4 exhibit many lethal defects, including impaired B-cell lymphopoiesis and lack of BM seeding by hematopoietic progenitors.16,17 Mutations in human CXCR4 lead to WHIM (warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis) syndrome, a combined immunodeficiency disease that is characterized by neutropenia as well as deficient B- and T-cell abundance and function.18 SDF-1 is also involved in proliferation and survival of various cells, including normal human CD34+ cells and pre-B ALL cells.19,20
SDF-1 regulates many interactions between primitive human CD34+ cells and the BM microenvironment. In particular, this ligand activates cell adhesion and transendothelial migration which is mediated by the major integrins very late antigen 4 (VLA-4), VLA-5, and lymphocyte function-associated antigen-1 (LFA-1).21,22 Transmigration of human leukemic cells through BM fibroblasts is also mediated by SDF-1/CXCR4 interactions, and the malignant cells use the β1 integrins VLA-4 and VLA-5.23 It has been shown that CXCR4 desensitization by pretreatment of human ALL cells with high levels of SDF-1 in vitro prior to their transplantation decreases their homing and engraftment levels in NOD/SCID mice that receive transplants.24
Chemokines can induce distinct signaling pathways that mediate cell growth, transcriptional activation, as well as cell motility. In vitro SDF-1–induced chemotaxis is inhibited by pertussis toxin (PTX), demonstrating that the 7 transmembrane receptor CXCR4 is coupled to Gαi proteins.11 Intracellular events induced by SDF-1 include elevation of cytoplasmic Ca2+ levels, activation of phosphoinositide 3-kinase (PI-3 kinase), and phosphorylation of mitogen-activated protein kinase kinase/extracellular signal regulated kinase (MEK/ERK) in several cell types.25-27
Actin cytoskeleton organization, a vital part of cell migration, has been shown to be regulated by the Rho proteins Rho, Rac, and Cdc42. These proteins belong to the Ras superfamily of small guanosine triphosphate (GTP) binding proteins and function as key regulators of many essential cellular processes, including actin cytoskeleton organization, gene transcription, and cell adhesion.28 Cdc42 has been shown to be involved in SDF-1–induced T-cell chemotaxis.29
In the present study, we investigated the role of SDF-1/CXCR4 interactions in the migration (both in vitro and in vivo) of human precursor-B ALL cells, as well as the integrins and signaling pathways activated on SDF-1 stimulation. The signaling pathways involved in the migration of the precursor-B ALL cells in comparison to CD34+ cells were examined, revealing both similarities as well as differences between normal and leukemic progenitor cells in their CXCR4 expression, activation, and SDF-1–mediated directional migration.
Materials and methods
Cells
All human cells were obtained after informed consent and were used in accordance with the procedures approved by the human experimentation and ethics committees of the Weizmann Institute. Precursor-B ALL cell lines Nalm-6 (kindly provided by Prof H. Ben-Bassat, Hadassah Medical School, Jerusalem, Israel), A1, B1, G2, and BRE (kindly provided by Prof M. Freedman, The Hospital for Sick Children, Toronto, ON, Canada) were derived from freshly obtained peripheral blood composed primarily of blast cells from children with poor prognosis B-lineage ALL.30 Cells were grown in Iscove modified Dulbecco medium (IMDM) (Biological Industries, Beit Haemek, Israel) supplemented with 10% fetal calf serum (Biological Industries), l-glutamine (Biological Industries), and penicillin and streptomycin antibiotics (GibcoBRL, Grand Island, NY). Leukemic cells from patients who had been newly diagnosed (Table 1) were diluted in phosphate-buffered saline (PBS) and centrifuged on Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden). Washed mononuclear cells (MNCs) were then cryopreserved in 10% dimethylsulfoxide, 40% fetal calf serum, and IMDM prior to use. Human cord blood (CB) samples from full-term deliveries were diluted 1:1 in PBS without Mg2+/Ca2+. Low-density MNCs were collected after standard separation on Ficoll-Paque (Amersham Pharmacia Biotech) and washed in PBS. Granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood (MPB) cells were obtained from healthy donors for clinical transplantation. CD34+ cells were purified by using the magnetic activated cell sorting (MACS) cell isolation kit and AutoMACS magnetic cell sorter (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions, and they were incubated overnight in medium supplemented with 50 ng/mL stem cell factor.
Mice
NOD/SCID mice (NOD/LtSz Prkdcscid)31 were bred and maintained under defined flora conditions in individually ventilated (high-efficiency particle-arresting filtered air) sterile microisolator cages (Techniplast, Varese, Italy) at the Weizmann Institute. All the experiments were approved by the animal care committee of the Weizmann Institute. In some experiments the 8-week-old mice were irradiated with a sublethal dose of 375 cGy from a cobalt source prior to transplantation. Leukemic cells (5-20 × 106 cells) or CD34+ cells (5 × 105 cells) were injected into the tail vein of irradiated (24 hours after irradiation) or nonirradiated mice. For in vivo blocking experiments, the cells were first preincubated for 30 minutes with 3 μg/106 cells of a blocking mouse antihuman VLA-4 (MCA697), anti–VLA-5 (MCA1187), anti–LFA-1 (MCA1149) (Serotec, Oxford, United Kingdom), or anti-CXCR4 monoclonal antibody (mAb; clone 12G5; Pharmingen, San Diego, CA) without washing. In other homing experiments, cells were pretreated with SDF-1α (1 μg/mL, 20 hours, 37°C; PeproTech, Rocky Hill, NJ), 100 ng/mL Clostridium difficile toxin B-10463 (Tox-B; 100 ng/mL, 20 hours, 37°C; kindly provided by Dr Aktories, Universitat Freiburg, Freiburg, Germany), PTX (100 ng/mL, 2 hours, 37°C), or chelerythrine chloride (CC; 10 μM, 30-60 minutes; Calbiochem, San Diego, CA) prior to injection. Cells were recovered from the BM of the mice 16 hours after transplantation, MNCs were counted, and the presence of human cells was detected by flow cytometry.
Flow cytometry analysis
Flow cytometry analysis was done as previously described.32 BM cells from mice that received transplants were flushed and resuspended in fluorescence activated cell sorting (FACS) buffer (PBS with 0.1% bovine serum albumin [BSA], and 0.02% sodium azide). Cells were stained with human-specific direct-labeled antibodies, 10 μL/mL purified antimouse CD16/CD32 Fc receptor (PharMingen), and 1% human plasma and incubated for 30 minutes at 4°C. Human cells and murine BM cells from mice not receiving transplants were used as a positive and negative control, respectively. The presence of human precursor-B ALL cells was identified by staining with anti-CD45–FITC (Immuno Quality Products, Groningen, The Netherlands), anti-CD10–FITC, or anti-CD19–FITC (Becton Dickinson, San Jose, CA) antibodies. Human CD34+ cells were detected in the BM by using human-specific anti-CD34–FITC (Becton Dickinson) and anti-CD38–phycoerythrin (PE; Beckman Coulter, Brea, CA) antibodies. The level of CXCR4 expression on cells was detected with PE-conjugated anti-CXCR4 antibodies (PharMingen). After staining, cells were washed in FACS buffer and analyzed by flow cytometry (FACSCalibur and CellQuest software; Becton Dickinson). When cells were stained for integrin expression, nonconjugated antibodies against VLA-4, VLA-5, and LFA-1 (Serotec) were used, and they were detected by using secondary FITC-conjugated F(ab′)2 fragment goat antimouse immunoglobulin G (IgG) (Jackson, West Grove, PA). In experiments testing homing and migration of normal precursor-B cells, CB MNCs were triple-stained with CD45-allophycocyanin (APC), CD10-FITC, and CD19-PE (Becton Dickinson). Human leukocytes were gated according to their expression of the pan-leukocyte marker CD45, and among this population the number of CD10+/CD19+ precursor-B cells was determined.
Chemotaxis assays
Chemotaxis experiments were assayed by using transwells (6.5-mm diameter, 5-μm pore) (Corning, Corning, NY) as previously described.22 Human cells (1-2 × 105) suspended in 100 μL medium were added to the upper chamber, and 600 μL medium with or without SDF-1 (10-1000 ng/mL) (PeproTech) was placed in the bottom chamber. After 4 hours at 37°C, migrating (bottom chamber) cells were counted for 30 seconds with the use of a FACSCalibur (Becton Dickinson). In some experiments, 1 to 2 × 106 cells/mL was preincubated (30 minutes at 4°C) in 100 μL medium containing either 5 μg control isotype-matching mAb (Becton Dickinson) or murine mAb specific to human CXCR4 (12G5 clone; Pharmingen). In other experiments, 1 to 2 × 106 cells/mL was preincubated in medium containing PTX (100 ng/mL, 2 hours, 37°C), CC (2-10 μM, 30-60 minutes, 37°C), or the CXCR4 antagonists T22 and T140 (kindly provided by Prof N. Fujii, Kyoto University, Kyoto, Japan) (1 and 10 μM, respectively, 1 hour, 37°C), with appropriate controls. When the effect of SDF-1 desensitization or Tox-B was tested, cells were preincubated for 20 hours with 1 μg/mL SDF-1 or 100 ng/mL Tox-B prior to migration.
Intracellular CXCR4 staining
Intracellular CXCR4 staining was done as previously described.33 In brief, CXCR4 expressed on the cell surface was blocked with nonconjugated antihuman CXCR4 mAb (clone 12G5, 10 μg/mL, 1 hour, 4°C). Cells were fixed with paraformaldehyde (4%, 20 minutes at room temperature; BDH, Poole, England) and then permeabilized with Triton X-100 (0.5%, 10 minutes at room temperature; Sigma, St Louis, MO). Anti-CXCR4–PE mAb was used to label the cells for flow cytometry for 30 minutes, 4°C. The cells were washed with PBS after each step.
Gelatin zymography
Normal CB CD34+ cells or precursor-B ALL cell lines (B1, G2, Nalm-6) were incubated in serum-free RPMI (2 × 106 cells/mL) with or without SDF-1 (1, 10, or 500 ng/mL) at 37°C for 40 hours as described elsewhere.34 Cell-conditioned media was then collected for zymographic analysis of matrix metalloproteinase 2 (MMP-2) and MMP-9 activity. Media samples were mixed with nonreducing sample buffer and loaded on 10% sodium dodecyl sulfate (SDS)–polyacrylamide gel copolymerized with 1 mg/mL gelatin derived from porcine skin (Sigma). After electrophoresis, gels were washed clear of SDS for 30 minutes in 2.5% Triton X-100, washed 3 times with H2O, and incubated at 37°C for 16 hours in developing buffer (50 mM Tris (tris(hydroxymethyl)aminomethane) pH = 8, 5 mM CaCl2, 200 mM NaCl, and 0.02% Brij). The gels were then stained with 0.25% Coomassie Brilliant Blue and destained (5% acetic acid, 10% MeOH) until clear bands appeared, indicating the presence of MMP-2 and MMP-9. Conditioned media from HT-1080 cells secreting both MMP-2 and MMP-9 served as control.
Statistics
Results of experimental points are reported as mean ± SE. Statistical significance was determined by Student t test for differences in means.
Results
Migration and homing of precursor-B ALL cells are dependent on CXCR4/SDF-1 interactions
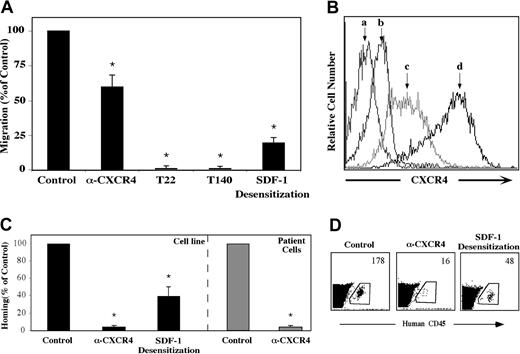
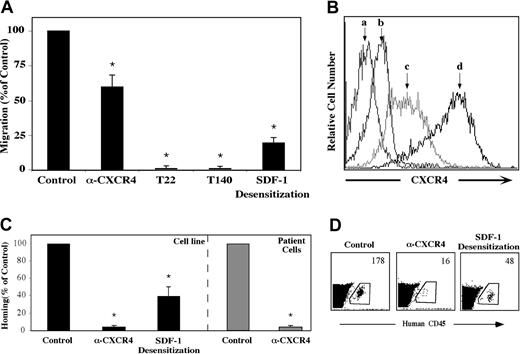
The crucial role of SDF-1 and CXCR4 in the homing and engraftment of human CD34+ progenitor cells, including CD38-/low SRCs has been shown in our lab.8,13,15 We set forth to determine whether a similar mechanism is involved in the migration and homing of precursor-B ALL cells. First, the capacity of precursor-B ALL cells to respond to SDF-1 in vitro was examined. All precursor-B ALL cell line cells tested expressed CXCR4 and migrated through transwell filters toward SDF-1 placed in the bottom chamber. Incubation with high concentrations (1 μg/mL) of SDF-1 leading to receptor desensitization inhibited migration by 80%, and the neutralizing monoclonal anti-CXCR4 12G5 antibody decreased in vitro migration by 40%. Complete inhibition of in vitro migration was achieved when G2 or Nalm-6 cells were preincubated with the CXCR4 peptide antagonists T22 and T140 (Figure 1A). As can be seen in Figure 1B, there is a strong correlation between CXCR4 expression level and the ability of the cells to migrate. Whereas untreated G2 cells express high levels of CXCR4 on their membrane, SDF-1 desensitization caused a significant decrease in CXCR4 levels. Pretreatment with the T22 peptide inhibitor caused an even stronger internalization from the membrane, which may explain the total inhibition of directional migration.
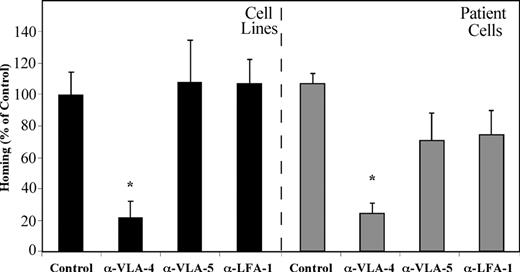
Migration and homing of precursor-B ALL cells depend on SDF-1/CXCR4 interactions. (A) Results show average percentage ± SE of in vitro migration of untreated (control), pretreated (α-CXCR4, T22, or T140) or SDF-1–desensitized (20 hours in IMDM containing 1 μg/mL SDF-1) Nalm-6 and G2 cells to 125 ng/mL SDF-1 (at least 3 experiments for each cell line). (B) Cell surface CXCR4 expression levels of unlabeled (a), T22 pretreated (b), SDF-1–desensitized (c), or untreated (d) G2 cells were ascertained. (C) Cells (5-20 × 106) from patients with newly diagnosed precursor-B ALL (patient nos. 1-5) or cell lines were injected into NOD/SCID mice either untreated, after blocking with anti-CXCR4–neutralizing antibodies (Nalm-6, G2, A1, BRE), or after 20 hours of incubation with 1 μg/mL SDF-1 (Nalm-6 and G2). Results show percentage of homing of human cells to the BM 16 hours after transplantation relative to control untreated cells (= 100%). Three or more mice were used for each cell line in each treatment. Cells from noninjected mice were used as a negative control. (D) A representative experiment showing homing of G2 cells. The number represents the number of human CD45+ cells per 106 acquired cells. *P < .05 compared with control.
Migration and homing of precursor-B ALL cells depend on SDF-1/CXCR4 interactions. (A) Results show average percentage ± SE of in vitro migration of untreated (control), pretreated (α-CXCR4, T22, or T140) or SDF-1–desensitized (20 hours in IMDM containing 1 μg/mL SDF-1) Nalm-6 and G2 cells to 125 ng/mL SDF-1 (at least 3 experiments for each cell line). (B) Cell surface CXCR4 expression levels of unlabeled (a), T22 pretreated (b), SDF-1–desensitized (c), or untreated (d) G2 cells were ascertained. (C) Cells (5-20 × 106) from patients with newly diagnosed precursor-B ALL (patient nos. 1-5) or cell lines were injected into NOD/SCID mice either untreated, after blocking with anti-CXCR4–neutralizing antibodies (Nalm-6, G2, A1, BRE), or after 20 hours of incubation with 1 μg/mL SDF-1 (Nalm-6 and G2). Results show percentage of homing of human cells to the BM 16 hours after transplantation relative to control untreated cells (= 100%). Three or more mice were used for each cell line in each treatment. Cells from noninjected mice were used as a negative control. (D) A representative experiment showing homing of G2 cells. The number represents the number of human CD45+ cells per 106 acquired cells. *P < .05 compared with control.
We found that similar to migration, homing of precursor-B ALL cells (both cell line and newly diagnosed patient cells) to the BM of NOD/SCID mice was also dependent on SDF-1/CXCR4 interactions. Homing of precursor-B ALL cell line cells to the BM was significantly reduced by 60% after SDF-1 desensitization and an even greater reduction (90%) was observed following pretreatment of the cells with neutralizing anti-CXCR4 antibodies (Figure 1C-D). Similarly, cells from patients with newly diagnosed precursor-B ALL that were incubated with neutralizing anti-CXCR4 antibodies prior to injection showed complete inhibition in homing to the BM of nonirradiated NOD/SCID mice (Figure 1C). All together these results demonstrate that CXCR4 signaling plays a crucial role in SDF-1–mediated directional migration and homing of precursor-B ALL cells.
Precursor-B ALL and normal CD34+ cells exhibit different expressions of CXCR4, SDF-1–induced migration, and need for irradiation of recipient mice
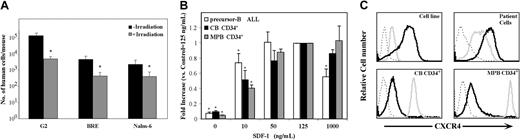
Although sublethal irradiation prior to cell injection is essential for optimal homing and engraftment of normal human CD34+ cells to hematopoietic organs of NOD/SCID mice,8,35 it was previously shown that sublethal irradiation is not a prerequisite for precursor-B ALL engraftment of NOD/SCID mice.5 Engraftment is composed of several distinct stages, first homing to the BM microenvironment, followed by retention and repopulation. We investigated whether irradiation is important for homing, the first crucial step of engraftment, by comparing the homing ability of precursor-B ALL cells into the BM of irradiated versus nonirradiated mice. Irradiation was not needed for successful homing of precursor-B ALL cell line cells. Moreover, a 15-fold increase in the total number of cells that homed to the BM was detected when NOD/SCID recipient mice were not preconditioned by total body irradiation (TBI) (Figure 2A, log scale).
Differences in CXCR4 expression, SDF-1–induced migration, and irradiation requirement between precursor-B ALL and CD34+ cells. (A) Precursor-B ALL cell line cells (5-20 × 106) were injected into NOD/SCID mice, either untreated (▪) or 48 hours after total body irradiation (375 cGy; ▦). The total number (average ± SE of at least 3 experiments) of human cells that homed to the murine BM 16 hours after transplantation is shown. This was calculated according to the number of human cells per 106 MNCs acquired by flow cytometry multiplied by the total number of MNCs in the BM. *P < .05 compared with homing into nonirradiated mice. (B) Fold increase (compared with migration to 125 ng/mL SDF-1) of spontaneous or SDF-1–induced migration (at the indicated concentrations) of precursor-B ALL (□; G2, Nalm-6, BRE cells; average ± SE of at least 3 experiments performed in duplicates for each cell line) and normal CB (▪) or MPB (▦) CD34+ cells. *P < .05 relative to migration toward 125 ng/mL SDF-1. (C) Immunofluorescence detection of intracellular (gray line), cell surface (black line), or isotype control (dotted line) expression of CXCR4 levels of normal CD34+ cells, G2 cells, or precursor-B ALL cells from patients with newly diagnosed disease (patient nos. 1, 3, 4, and 5). Representative experiments of at least 3 independent experiments for each group are shown.
Differences in CXCR4 expression, SDF-1–induced migration, and irradiation requirement between precursor-B ALL and CD34+ cells. (A) Precursor-B ALL cell line cells (5-20 × 106) were injected into NOD/SCID mice, either untreated (▪) or 48 hours after total body irradiation (375 cGy; ▦). The total number (average ± SE of at least 3 experiments) of human cells that homed to the murine BM 16 hours after transplantation is shown. This was calculated according to the number of human cells per 106 MNCs acquired by flow cytometry multiplied by the total number of MNCs in the BM. *P < .05 compared with homing into nonirradiated mice. (B) Fold increase (compared with migration to 125 ng/mL SDF-1) of spontaneous or SDF-1–induced migration (at the indicated concentrations) of precursor-B ALL (□; G2, Nalm-6, BRE cells; average ± SE of at least 3 experiments performed in duplicates for each cell line) and normal CB (▪) or MPB (▦) CD34+ cells. *P < .05 relative to migration toward 125 ng/mL SDF-1. (C) Immunofluorescence detection of intracellular (gray line), cell surface (black line), or isotype control (dotted line) expression of CXCR4 levels of normal CD34+ cells, G2 cells, or precursor-B ALL cells from patients with newly diagnosed disease (patient nos. 1, 3, 4, and 5). Representative experiments of at least 3 independent experiments for each group are shown.
These results might be explained by increased sensitivity to SDF-1; therefore in vitro migration of precursor-B ALL cell line cells toward different concentrations of SDF-1 was tested. We found that, although migration capacity of precursor-B ALL cells reached high and maximal levels already at low levels of SDF-1 (10 and 50 ng/mL, respectively), a significant decrease was detected in the migration toward very high levels of SDF-1 (1 μg/mL). In contrast, migration of normal CD34+ cells (both CB and MPB) reached peak levels of migration only at higher concentrations of SDF-1 (125 ng/mL), and no significant decrease was detected when SDF-1 levels were further increased (Figure 2B).
To understand these differences in the response to SDF-1, the level of expression of intracellular and cell surface CXCR4 were checked. Precursor-B ALL cells expressed high levels of cell surface CXCR4, yet only low levels of intracellular CXCR4 were detected. In contrast, in normal CB and MPB CD34+ cells most of the CXCR4 molecules were localized within the cells, and only a small part of the receptors were expressed on the membrane surface (Figure 2C). These differences in the expression levels of intracellular and cell surface CXCR4 suggest different CXCR4 regulation in normal and malignant human progenitor cells and may explain different cell behavior in response to SDF-1.
Different patterns of MMP secretion by the precursor-B ALL and CB CD34+ cells
Matrix metalloproteinases (MMPs) are proteolytic enzymes secreted by normal and malignant cells and have been shown to play a crucial role in cell motility and invasion through the extracellular matrix (ECM).36 SDF-1 induces expression and secretion of MMP-2 and MMP-9 by normal CD34+ cells, which, in turn, can inactivate this chemokine.34,37 To determine the role of MMPs in the motility of the normal and leukemic progenitors, the levels of MMP-2 and MMP-9 secretion were assayed by zymography. CB CD34+ cells and precursor-B ALL cells exhibited a different pattern of MMP secretion. Whereas normal CB CD34+ cells secreted MMP-9 but only low levels of MMP-2, precursor-B ALL cell line cells (B1, G2, and Nalm-6) secreted mainly MMP-2 and only low or undetectable levels of MMP-9. Furthermore, stimulation of the leukemic cells with SDF-1 at different concentrations had no effect on MMP secretion. This finding is in contrast to CD34+ cells in which MMP secretion is enhanced in response to high levels of SDF-134 (Figure 3). The different patterns of MMP secretion exhibited by the 2 cell populations may explain the differences in their ability to migrate and home into the BM.
Different pattern of MMP secretion by precursor-B ALL and CB CD34+ cells. CB CD34+ and precursor-B ALL cells were cultured for 40 hours with or without SDF-1. The presence of MMP-2 and MMP-9 in the conditioned media was detected by gelatin zymography. Conditioned media from HT-1080 cells secreting both MMP-2 and MMP-9 served as control. A representative of 3 independent experiments conducted is shown.
Different pattern of MMP secretion by precursor-B ALL and CB CD34+ cells. CB CD34+ and precursor-B ALL cells were cultured for 40 hours with or without SDF-1. The presence of MMP-2 and MMP-9 in the conditioned media was detected by gelatin zymography. Conditioned media from HT-1080 cells secreting both MMP-2 and MMP-9 served as control. A representative of 3 independent experiments conducted is shown.
Role of adhesion molecules in homing of precursor-B ALLand normal CB CD34+ cells
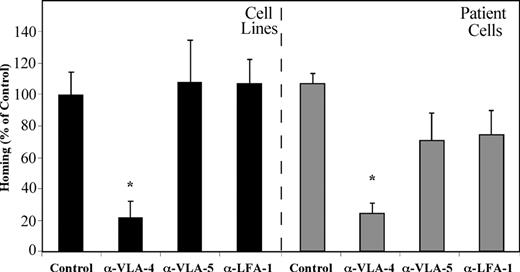
Bradstock et al23 have demonstrated that ALL blasts migrate into layers of BM fibroblasts in vitro by using the β1 integrins VLA-4 and VLA-5. We have shown that homing of normal human CB CD34+ cells into the BM of NOD/SCID mice is equally dependent on VLA-4, VLA-5, and LFA-1.15 We set out to test the direct effects of β1 and β2 integrins on the homing of precursor-B ALL cells to the BM of NOD/SCID mice. Precursor-B ALL cells were treated with neutralizing antibodies against each of the integrins separately before transplantation. Homing of precursor-B ALL cell line cells, as well as cells from patients with newly diagnosed precursor-B ALL into the BM was significantly reduced by blocking VLA-4 (80% and 85%, respectively) compared with nontreated cells. Pretreatment of cells with anti–VLA-5 or anti–LFA-1 antibodies did not inhibit homing of precursor-B ALL cell line cells, and a 40% and 45% (respectively) decrease in homing was detected when patient cells were injected. Altogether, homing of cells after pretreatment with VLA-4–neutralizing antibodies was significantly lower than that of VLA-5 and LFA-1 pretreated cells (Figure 4). These results demonstrate that, although all 3 integrins play an equally important role in the homing of normal CD34+ cells, activation of VLA-4 is the most prominent in homing of precursor-B ALL cells.
VLA-4 has the most prominent role in mediating homing of precursor-B ALL cells. Precursor-B ALL cells were pretreated with specific neutralizing antibodies for the specified integrins prior to injection into NOD/SCID mice. Results show percentage (compared with control = 100%) ± SE of human cells recovered from the BM of injected mice and are the average of 3 cell lines (A-1, G2, BRE) or 5 patient cells (patient nos. 1-5). Each group contains 6 or more mice. *P < .05 compared with both control as well as α-VLA-5 and α-LFA-1 pretreatment.
VLA-4 has the most prominent role in mediating homing of precursor-B ALL cells. Precursor-B ALL cells were pretreated with specific neutralizing antibodies for the specified integrins prior to injection into NOD/SCID mice. Results show percentage (compared with control = 100%) ± SE of human cells recovered from the BM of injected mice and are the average of 3 cell lines (A-1, G2, BRE) or 5 patient cells (patient nos. 1-5). Each group contains 6 or more mice. *P < .05 compared with both control as well as α-VLA-5 and α-LFA-1 pretreatment.
Involvement of key signaling molecules in SDF-1–induced cell motility
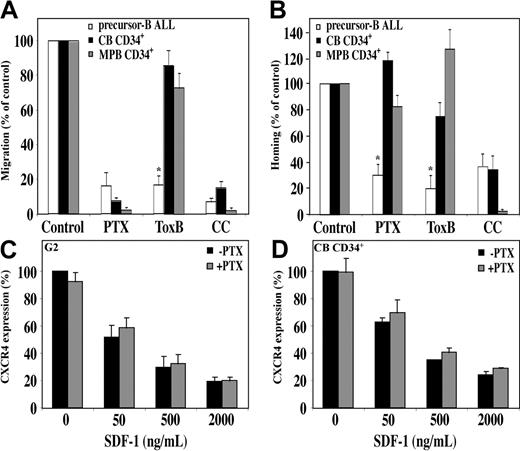
The chemokine receptor CXCR4 is a G-protein–coupled receptor. PTX, an inhibitor of signal transduction mediated by the Gαi subunit, almost completely abrogates in vitro migration of normal human CD34+ cells.11,15 Similarly, we found that in vitro migration of precursor-B ALL cells toward a gradient of SDF-1 was also inhibited by PTX. In addition, homing of precursor-B ALL cells was inhibited by 70% after pretreatment with PTX; however, only a small and not significant decrease was detected in homing of MPB CD34+ cells, and a slight increase was detected in homing of CB CD34+ cells following pretreatment with PTX15 (Figure 5A-B). The distinct response to PTX between the normal and leukemic cells is not due to differences in receptor internalization, because SDF-1–induced CXCR4 internalization was not affected by PTX pretreatment in both the normal and the malignant cells (Figure 5C-D). In addition, PTX inhibited the SDF-1–induced calcium mobilization in both normal and malignant cells in a similar manner (data not shown). This finding is in accordance with a previous report by Amara et al38 that showed Gαi-independent CXCR4 internalization in T cells.
SDF-1/CXCR4 interactions activate different signaling pathways in migration and homing of precursor-B ALL and CD34+ cells. Migration and homing of cells following pretreatment with pertussis toxin (PTX; 100 ng/mL, 2 hours, 37°C), Toxin B (ToxB; 100 ng/mL, 20 hours, 37°C), or chelerythrine chloride (CC; 10 μM, 30-60 minutes, 37°C) were tested. (A) Results show average percentage ± SE of in vitro migration of pretreated human precursor-B ALL, CB CD34+, and MPB CD34+ cells compared with control untreated cells (= 100%). Results of migration of precursor-B ALL cells represent average of G2, BRE, and Nalm-6 cells (at least 3 experiments for each cell line). (B) Results show percentage of homing of pretreated human precursor-B ALL, CB, and MPB CD34+ cells to the BM 16 hours after transplantation compared with control untreated cells (= 100%). Results represent average ± SE of cell lines G2, B-1, and A-1, as well as cells from patient nos. 1, 2, 3, 4, and 6. Cell viability was greater than 90% before injection. *P < .05 in comparison of precursor-B ALL with normal CD34+ cells for each treatment. G2 cells (C) or CB CD34+ cells (D) were incubated with or without PTX (100 ng/mL) for 2 hours and then stimulated with increasing concentrations of SDF-1. Surface expression of CXCR4 was determined by flow cytometry. Results show average of 3 independent experiments compared with control cells not stimulated with SDF-1 (= 100%).
SDF-1/CXCR4 interactions activate different signaling pathways in migration and homing of precursor-B ALL and CD34+ cells. Migration and homing of cells following pretreatment with pertussis toxin (PTX; 100 ng/mL, 2 hours, 37°C), Toxin B (ToxB; 100 ng/mL, 20 hours, 37°C), or chelerythrine chloride (CC; 10 μM, 30-60 minutes, 37°C) were tested. (A) Results show average percentage ± SE of in vitro migration of pretreated human precursor-B ALL, CB CD34+, and MPB CD34+ cells compared with control untreated cells (= 100%). Results of migration of precursor-B ALL cells represent average of G2, BRE, and Nalm-6 cells (at least 3 experiments for each cell line). (B) Results show percentage of homing of pretreated human precursor-B ALL, CB, and MPB CD34+ cells to the BM 16 hours after transplantation compared with control untreated cells (= 100%). Results represent average ± SE of cell lines G2, B-1, and A-1, as well as cells from patient nos. 1, 2, 3, 4, and 6. Cell viability was greater than 90% before injection. *P < .05 in comparison of precursor-B ALL with normal CD34+ cells for each treatment. G2 cells (C) or CB CD34+ cells (D) were incubated with or without PTX (100 ng/mL) for 2 hours and then stimulated with increasing concentrations of SDF-1. Surface expression of CXCR4 was determined by flow cytometry. Results show average of 3 independent experiments compared with control cells not stimulated with SDF-1 (= 100%).
Rho proteins are key regulators of signal transduction and mediate actin cytoskeleton rearrangement and cell motility.28 We tested whether these proteins have a role in SDF-1–induced hematopoietic cell migration. Pretreatment of precursor-B ALL cells with Toxin B, a single-chained 270-kDa molecule that specifically inactivates the Rho proteins RhoA, Cdc42, and Rac, resulted in a significant reduction in migration and homing of the cells. In vitro migration was inhibited by 85%, and a 90% reduction in homing to the BM was detected (Figure 5A-B). Contrary to that, when normal CB or MPB CD34+ progenitor cells were preincubated with Toxin B, only a slight decrease (15% and 25%, respectively) in migration was observed. Similarly, homing of CB CD34+ into the BM of NOD/SCID mice was reduced by only 25%, and a 25% increase in homing of MPB CD34+ cells was documented (Figure 5A-B).
Laudanna et al39 found that CC, a broad-range protein kinase C (PKC) inhibitor, blocks both CXCR2-mediated adhesion and chemotaxis of neutrophils while studying another CXC chemokine, IL-8. We found that the PKC pathway is also involved in SDF-1/CXCR4 signaling because CC significantly inhibited SDF-1–induced migration of both normal CD34+ cells and precursor-B ALL cells. Moreover, pretreatment of human CB CD34+ and precursor-B ALL cells with CC efficiently inhibited the homing of 65% of the cells into the BM15 (Figure 5A-B). Interestingly, both homing and migration of MPB CD34+ cells were totally inhibited following pretreatment of cells with CC. Taken together these results demonstrate that SDF-1 stimulation leads to activation of different signaling pathways with only a partial overlap in normal and malignant human progenitors.
Unique SDF-1–induced signaling pathways result from the leukemic phenotype of precursor-B ALL cells
The differences between normal human CD34+ cells and malignant precursor-B ALL cells may result from the fact that the precursor-B cells are more differentiated than the CD34+ cells. Alternatively, the differences may be due to a leukemic “phenotype” acquired by the malignant cells. To address this issue, SDF-1–induced migration and homing of CB MNCs following pretreatment with signaling inhibitors was tested. Human leukocytes were gated according to their expression of CD45, and among this population the number of CD10+/CD19+ precursor-B cells was determined. Similar to migration of CD34+ cells, SDF-1–induced migration of normal precursor-B CD10+/CD19+ population of CB MNCs was inhibited by PTX but was only slightly reduced following treatment with Toxin B. Unlike the leukemic cells, yet similar to normal CD34+ cells, pretreatment with PTX resulted in a slight increase in homing of normal precursor-B cells (Figure 6). Thus, normal CD10+/CD19+ precursor-B cells share similar signaling pathways with normal CD34+ cells but differ from those of the leukemic cells, strongly suggesting that the unique response to SDF-1 results from changes acquired by the malignant cells and not differences between primitive precursors and B-lineage progenitors.
Signaling inhibitors affect normal precursor-B cells similar to CD34+ cells. CB MNCs were pretreated with pertussis toxin (PTX; 100 ng/mL, 2 hours, 37°C), Toxin B (ToxB; 100 ng/mL, 20 hours, 37°C), or chelerythrine chloride (CC; 10 μM, 30-60 minutes, 37°C) prior to in vitro migration or homing into the BM of NOD/SCID mice. Cells that migrated or homed were gated according to their expression of CD45, and among this population the number of CD10+/CD19+ precursor-B cells was determined. Results show average (of at least 3 experiments) percentage ± SE of in vitro migration and homing of normal precursor-B CD10+/CD19+ cells relative to control untreated cells (= 100%). *P < .05.
Signaling inhibitors affect normal precursor-B cells similar to CD34+ cells. CB MNCs were pretreated with pertussis toxin (PTX; 100 ng/mL, 2 hours, 37°C), Toxin B (ToxB; 100 ng/mL, 20 hours, 37°C), or chelerythrine chloride (CC; 10 μM, 30-60 minutes, 37°C) prior to in vitro migration or homing into the BM of NOD/SCID mice. Cells that migrated or homed were gated according to their expression of CD45, and among this population the number of CD10+/CD19+ precursor-B cells was determined. Results show average (of at least 3 experiments) percentage ± SE of in vitro migration and homing of normal precursor-B CD10+/CD19+ cells relative to control untreated cells (= 100%). *P < .05.
Discussion
Precursor-B ALL is the most common childhood malignancy and the second most common adult acute leukemia.40-42 The leukemic cells have the ability to infiltrate the liver, spleen, lymph nodes, and central nervous system. Investigating the mechanisms of extramedullary dissemination of the leukemic cells is extremely important, because specific targeting of these cells may improve treatment of patients. Immune deficient SCID and NOD/SCID mice provide a useful preclinical model to study in vivo malignant migration and repopulation by human precursor-B ALL progenitors, because this functional system can predict the patient's prognosis.3,5,6 In the present study we demonstrate that the migration and homing requirements of malignant precursor-B ALL cells differ from those required for successful homing and migration of normal CD34+ progenitors despite the dependence of both cell populations on SDF-1/CXCR4 interactions.
High expression of CXCR4 by the leukemic cells is strongly predictive for extramedullary organ invasiveness, including infiltration to the central nervous system in patients with childhood ALL.43 We show that, similar to normal CD34+ progenitor cells, SDF-1/CXCR4 interactions are essential for both in vitro migration as well as in vivo homing of malignant precursor-B ALL cells. Pretreatment leading to interference of SDF-1/CXCR4 interactions demonstrates a strong correlation between receptor expression and migratory capacity both in vitro and in vivo. These results are in accordance with a report by Shen et al24 that showed reduction in homing and engraftment of precursor-B ALL cells following CXCR4 desensitization by pretreatment with high concentrations of SDF-1.
Internal reservoirs of CXCR4 are of utmost importance for cell motility and development, and we have recently shown that rapid and dynamic turnover between intracellular and cell surface expression is responsible for homing and engraftment by normal CB CD34+/CXCR4- sorted cells.33 We reveal a major difference in the ratio of intracellular versus cell surface CXCR4 expression obtained by the leukemic cells and normal CD34+ (both CB and MPB) cells. The latter express relatively low levels of cell surface CXCR4 in comparison to high intracellular levels. Contrary to that, the leukemic cells express high levels of cell surface CXCR4 but only low levels of intracellular receptor. The enhanced expression of cell surface CXCR4 by the leukemic cells may explain their unique response to low levels of SDF-1 in both migration and homing into nonirradiated recipient mice. Eventually, this may also lead to the rapid proliferation of the malignant cells, since preliminary results indicate that the leukemic cells exhibit increased proliferation and survival only in response to low levels of SDF-1. This is in contrast to normal human CD34+ progenitor cells whose survival has been shown to be supported only by high levels (100 ng/mL) of SDF-1 but not by lower levels.19
Total body irradiation is widely used clinically as well as in experimental models as a crucial conditioning procedure preceding stem cell transplantation. Ballen et al35 have shown that following transplantation of CB MNCs, engraftment of the CD34+ population is increased when recipient mice were irradiated prior to injection. We show that precursor-B ALL cells do not require preconditioning of the host by sublethal irradiation to successfully home to the BM of NOD/SCID mice. Conditioning of mice with DNA-damaging agents such as ionizing irradiation causes an increase in SDF-1–expression and secretion by immature mesenchymal osteoblasts, adipocytes, fibroblasts, and endothelial cells within the BM.8 We suggest that, because of their increased sensitivity to SDF-1, the leukemic cells exhibit unique homing abilities and are able to home to the BM of nonirradiated mice, in which SDF-1 levels are low. However, homing of the leukemic cells is reduced when mice are irradiated and SDF-1 levels are increased, probably because the leukemic cells have reduced migratory capacity toward high levels of SDF-1. Normal human CD34+ cells respond poorly to low concentrations of SDF-1 and, therefore, require irradiation that will increase SDF-1 levels in the BM of mice that received transplants.
MMP-2 and -9 secretion in response to SDF-1 has been shown to be involved in transmatrigel migration of immature human CD34+ cells as well as in stem cell mobilization and recruitment to injured murine liver, suggesting a possible role for MMPs in hematopoietic cell motility.34,44,45 We show that, unlike CB CD34+ cells, MMP secretion by the leukemic cells is high even without stimulation with SDF-1. This finding, together with the increased migration of the leukemic cells in response to relatively low concentrations of SDF-1, may explain the potential of the malignant cells to home into the BM of nonirradiated mice, because their ability to penetrate through the ECM is optimal even without stimulation with increased SDF-1 levels (due to irradiation). Furthermore, substrate specificity of the different enzymes may also account for the differences in homing capacities because MMP-2, which is secreted by the leukemic cells, has been shown to degrade fibronectin and various types of collagen that are not degraded by MMP-9 (secreted by the normal CD34+ cells).36
The major integrins VLA-4, VLA-5, and LFA-1 expressed by CD34+ cells are activated by SDF-1 and are necessary for successful engraftment of NOD/SCID mice.22 SDF-1 and CXCR4 are involved in regulation of β1 integrin function in precursor-B ALL cells.24 We found that VLA-4 has a unique and crucial role in the homing of precursor-B ALL cells to the BM; however, homing of normal CB CD34+ cells is equally dependent on all 3 major integrins.15 A distinct role for the different integrins may also explain the need for TBI in the homing process of normal progenitors, because it was recently shown that irradiation alters the expression of adhesion molecules on the lumen of BM microvessels.46
CXCR4 is a 7-transmembrane receptor coupled to a heterotrimeric GTP-binding protein. PTX is a known inhibitor of the Gαi subunit. We have previously shown that in vivo homing of normal CD34+ cells is not inhibited by PTX.15 However, we report here that both migration and homing of precursor-B cells were inhibited following pretreatment with PTX. Inhibition of in vitro migration in transwells but not homing of normal CD34+ cells by PTX remains an unresolved issue. One possible explanation is that different signaling pathways are activated in the 2 processes. Alternatively, it is also possible that homing of PTX-treated CD34+ cells is mediated by internal CXCR4. Indeed we have recently shown that homing is mediated by intracellular CXCR4 that can rapidly be expressed on the cell surface.33 Interestingly, PTX has been shown to up-regulate CXCR4 levels and cause mobilization of leukocytes from the BM to the peripheral blood.47 Preliminary results indicate that washing affects CXCR4 levels in PTX-pretreated CD34+ cells (data not shown). Therefore, it could be that on cell injection intracellular CXCR4 molecules in PTX-pretreated cells that are not affected by the inhibitor are relocated to the cell surface, thus enabling successful homing. If so, the low levels of internal reservoirs of CXCR4 might explain the inability of precursor-B ALL cells to home to the BM following treatment with PTX.
Rho GTPases control a wide variety of signal transduction pathways and have a pivotal role in regulating actin cytoskeleton rearrangement.28 We show that small GTP binding proteins from the Rho family are involved in the migration process of precursor-B ALL cells, because Toxin-B inhibited both in vitro migration and in vivo homing. However, migration and homing of normal CD34+ cells was significantly less affected by Toxin-B, indicating that different signaling pathways are activated in SDF-1-induced precursor-B ALL and immature CD34+ cell motility. The PKC signaling pathway was shown to be essential in the migration and adhesion of neutrophils induced by the CXC chemokine IL-8, because both activities were blocked in a dose-dependent manner by CC, a broad-range PKC inhibitor.39 CC inhibited the migration and homing of both CD34+ and precursor-B ALL cells in a similar manner, demonstrating a partial overlap in CXCR4-induced signaling in both populations involving the PKC pathway.
Our results demonstrate that in normal CD10+/CD19+ precursor-B cells the signaling pathways activated following stimulation with SDF-1 are similar to those activated in normal CD34+ cells but differ from those of leukemic precursor-B ALL cells. This finding indicates that the differences reported here are most probably based on changes acquired by the malignant cells and not merely differences between primitive multilineage precursors and B-lineage progenitors. In summary, we show that the mechanisms of migration and homing of malignant cells differ from those required for successful homing and migration of normal CD10+/CD19+ precursor B and progenitor CD34+ cells. This is reflected by differences in the preconditioning of recipient mice, response to various SDF-1 concentrations, cell surface, and intracellular levels of CXCR4, involvement of adhesion molecules and signaling pathways activated. These findings provide new insight on leukemic cell biology and may enable the development of novel therapeutic protocols aimed at preventing cancer cell proliferation and dissemination while only minimally affecting normal hematopoietic stem cells in patients with ALL.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-06-1891.
Supported in part by grants from the Minerva Foundation, Israel Cancer Research Fund (ICRF), and the Israel Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof M. Freedman (The Hospital for Sick Children, Toronto, Canada) for providing the cell lines A1, B1, BRE, and G2, and Prof H. Ben-Bassat and Mrs T. Shlomai (Hadassah Medical School, Jerusalem, Israel) for providing the Nalm-6 cells. We would also like to thank Prof R. Henschler and Dr K. Aktories for providing the Clostridium difficile toxin B-10463 inhibitor, and Prof N. Fujii for providing the CXCR4 antagonists T-22 and T-140.