Abstract
Four patients with hypereosinophilic syndrome (HES) refractory to or intolerant of treatment with conventional therapy were treated with a single 1 mg/kg dose of SCH55700. SCH55700 was extremely well tolerated. Two of the 4 patients responded with a fall in eosinophil counts to within the normal range within 48 hours of receiving the drug, accompanied by marked improvement in clinical signs and symptoms. Response was not predicted by serum interleukin-5 (IL-5) levels or presence of the FIP1L1/PDGFRA mutation. Eosinophil counts remained suppressed for up to 12 weeks after treatment; however, exacerbation of symptoms and eosinophilia above baseline levels occurred as drug levels waned. Reinstitution of treatment with monthly SCH55700 led to decreased eosinophilia and symptomatic improvement, albeit to a lesser degree than that seen after the initial dose. These data suggest that anti–IL-5 therapy may be useful in the treatment of HES irrespective of the underlying etiology, although the observed rebound eosinophilia and attenuation of response require further study. (Blood. 2004;103:2939-2941)
Introduction
Hypereosinophilic syndrome (HES) is a heterogeneous group of disorders characterized by the presence of unexplained eosinophilia (> 1.5 × 109/L [> 1500/mm3]) resulting in end organ damage. Although some patients with HES have clonal proliferation of lymphocytes1 or eosinophils,2 the etiology of the syndrome remains unknown in most. Regardless of the underlying etiology, the severity of the clinical pathology in HES is felt to reflect the extent of eosinophil activation in the tissues, and eosinophil granule proteins have been demonstrated in the serum and affected tissues of patients with HES.3
Whereas chemotherapeutic agents, including corticosteroids, hydroxyurea, and interferon-α, have demonstrated success in decreasing eosinophilia and end organ damage in some patients with HES, many patients become refractory to treatment and/or develop significant drug-related side effects.4 Although recent reports of the safety and efficacy of imatinib mesylate treatment of HES have been dramatic, this agent is only effective in a subset (approximately 50%) of patients with HES, and resistance has already been described.2 Consequently, new approaches to therapy are needed.
SCH55700 is a humanized rat monoclonal antihuman interleukin-5 (IL-5) antibody that has been shown to decrease peripheral blood eosinophilia in patients with asthma.5 Because eosinophils play a central role in the pathogenesis of HES, the aim of this study was to evaluate the safety and efficacy of SCH55700 in reducing peripheral blood eosinophilia and clinical symptoms in patients with HES.
Study design
Four subjects with HES refractory to or intolerant of therapy with corticosteroids, hydroxyurea, and interferon-α were enrolled in a pilot phase 1/2 study of single-dose SCH55700 (1 mg/kg intravenously; Schering-Plough Research Institute, Kenilworth, NJ). Their clinical characteristics are summarized in Table 1. Subjects with a known clonal hematopoietic process as determined by cytogenetics, flow cytometry, or polymerase chain reaction (PCR)–based T-cell receptor gamma (TCRγ) and immunoglobulin heavy chain gene rearrangement analysis were excluded from the study. The FIP1L1/PDGFRA fusion gene associated with sensitivity to imatinib in HES2 had not been described at the time of the study; hence, subjects with this abnormality were eligible for participation. The presence of the fusion transcript was subsequently assessed in all patients by nested reverse transcriptase–PCR of RNA isolated from peripheral blood mononuclear cells (PBMCs) as described previously.2
Subjects were monitored as inpatients for 72 hours following the first dose of SCH55700, and complete blood counts were performed daily for 3 days, weekly for 4 weeks, and monthly until the eosinophil count returned to baseline for 2 months. All laboratory testing reported in this study was performed in the Department of Laboratory Medicine at the National Institutes of Health (NIH) Clinical Center, with the exception of serum IL-5 levels, which were measured by Pierce Biotechnology (Rockford, IL) using a multiplex sandwich enzyme-linked immunosorbent assay (ELISA) as described previously,6 and serum levels of SCH55700, which were measured by ELISA at Schering-Plough Research Institute.5
Subjects who demonstrated a reduction in eosinophilia and evidence of clinical improvement in response to single-dose SCH55700 were eligible to receive 5 additional doses of SCH55700 (1 mg/kg intravenously) at monthly intervals. Bone marrow aspirate and biopsy were performed at baseline, one month after treatment, and prior to the last dose of SCH55700 in patients receiving monthly dosing.
The study was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board, and informed consent was obtained from all study participants.
Results and discussion
SCH55700 was extremely well tolerated. One patient complained of transient long-bone pain occurring 6 to 12 hours after the first and second infusions of SCH55700 that was similar in nature to what she had experienced previously with acute lowering of her eosinophil count with steroids. A low-grade fever and upper respiratory symptoms occurred within 3 days of the drug infusion on 2 separate occasions in a single patient and resolved without treatment. In both instances, other members of the patient's family were ill with similar symptoms. No other adverse effects were reported.
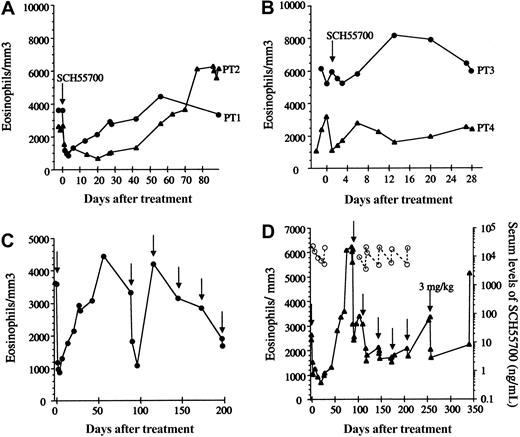
Patients 1 and 2 experienced a rapid decrease in eosinophilia in response to SCH55700 (Figure 1A) accompanied by marked improvement in their symptoms, including resolution of skin rash and mucosal ulcerations (patient 1), angioedema (patient 2), fatigue, myalgias, and arthralgias. The clinical and hematologic response was prolonged, lasting for more than 30 days following the infusion of SCH55700, but was associated with a rebound in the peripheral eosinophil count to levels above the pretreatment baseline and a severe exacerbation of symptoms in both subjects at 6 to 8 weeks after treatment. This rebound was preceded by a rise in serum IL-5 detectable at 1 month after treatment (Table 1).
Eosinophil response to SCH55700 in patients with HES. Absolute eosinophil count (closed symbols) as a function of time is shown prior to and following a single 1 mg/kg dose of SCH55700 (A-B). Absolute eosinophil counts prior to and following multiple-dose SCH55700 (1 mg/kg intravenously unless otherwise noted) are shown in panels C (patient 1) and D (patient 2). Arrows indicate drug administration. Serum levels of SCH55700 are represented by open symbols (D).
Eosinophil response to SCH55700 in patients with HES. Absolute eosinophil count (closed symbols) as a function of time is shown prior to and following a single 1 mg/kg dose of SCH55700 (A-B). Absolute eosinophil counts prior to and following multiple-dose SCH55700 (1 mg/kg intravenously unless otherwise noted) are shown in panels C (patient 1) and D (patient 2). Arrows indicate drug administration. Serum levels of SCH55700 are represented by open symbols (D).
Patient 3 had neither a decrease in his eosinophil count (Figure 1B) nor improvement in his symptoms following treatment with SCH55700 but experienced a self-limited exacerbation in symptoms and peripheral eosinophilia during weeks 1 to 4 after treatment. Patient 4 experienced a rapid decrease in her peripheral eosinophil count from 3.209 × 109/L (3209/mm3) to 1.105 × 109/L (1105/mm3) immediately following the infusion of SCH55700; however, her symptoms remained unchanged, and her eosinophil level returned to baseline within 7 days of drug administration (Figure 1B). Of note, this patient (patient 4) was the only subject with increased IL-5 levels at baseline (223 pg/mL). Although her serum IL-5 levels decreased following treatment, they remained detectable (14.5 pg/mL on day 3), suggesting that the dose of SCH55700 may have been inadequate in this patient.
Because of their dramatic response to single-dose therapy, patients 1 and 2 received 5 additional monthly doses of SCH55700 at 1 mg/kg per dose. Although both patients responded to the second dose of SCH55700 with a rapid decrease in eosinophilia (Figure 1C-D) and improvement in symptoms, the magnitude and duration of the improvement in symptoms and eosinophilia lessened with each subsequent dose. Drug levels remained detectable throughout treatment and rose with each infusion, inconsistent with the development of neutralizing antibodies as an explanation for the attenuation of response (Figure 1D). Bone marrow biopsy was performed prior to, at 1 month following treatment, and at the time of the last monthly dose. In contrast to studies in asthma that have demonstrated maturational arrest in the bone marrow following anti–IL-5 therapy,7 no significant changes in bone marrow cellularity or eosinophilia were observed (Table 1). Potential explanations for this discrepancy include differences in the underlying disease being studied (asthma versus HES), the antibodies used (mepolizumab versus SCH55700), the dosing (10 mg/kg versus 1 mg/kg), and the timing of the bone marrow sample (2 weeks versus 4 weeks after infusion).
The present study suggests that SCH55700 may be useful in treating a subset of patients with HES irrespective of the underlying etiology and independent of the presence of detectable serum IL-5 levels. Additional studies are needed to determine optimal dosing as well as the prevalence and mechanisms of the observed rebound eosinophilia and attenuation of response.
Supported by the intramural budget of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
One of the authors (T.P.H.) has declared a financial interest in Schering-Plough Research Institute, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-10-3620.
The authors thank Dr Yae Jean Kim for help with the processing of patient specimens and critical review of the manuscript. Schering-Plough Research Institute (SPRI) provided SCH55700.