Abstract
Mutational analysis of the c-kit gene in a patient with a previously undescribed variant of mast cell disease revealed a germline mutation, Phe522Cys, within the transmembrane portion of the Kit receptor protein. Transfection experiments revealed that the mutation caused ligand-independent autophosphorylation of Kit, which was inhibited by the tyrosine kinase inhibitor imatinib mesylate. The patient's bone marrow biopsy and aspirate displayed unique pathologic features with the presence of excessive numbers of mature-appearing mast cells and absence of aberrant mast cell surface expression of CD2, CD25, and CD35. Therapy with imatinib mesylate resulted in a dramatic improvement in mast cell burden and clinical symptoms. These results highlight the significance of the transmembrane region of Kit in activation of the molecule and its importance in mast cell development and suggest a role for screening for transmembrane c-kit mutations in patients with mastocytosis in association with the decision to use imatinib mesylate. (Blood. 2004;103:3222-3225)
Introduction
Systemic mastocytosis is a clonal neoplasm of the mast cell hematopoietic progenitor associated with gain-of-function mutations involving the tyrosine kinase domain of c-kit.1 There is no specific therapy to cure mast cell disease associated with c-kit tyrosine kinase domain mutations (most commonly Asp816Val) because these mutations confer resistance to the currently available tyrosine kinase inhibitor imatinib mesylate by interfering with the binding of the drug to the enzymatic site of the Kit molecule.2-4
During our efforts to screen for novel mutations in mast cell disease, we identified a point mutation within the transmembrane segment of Kit, which resulted in a substitution of a phenylalanine residue by a cysteine at codon 522 in a patient with systemic mastocytosis. This is the first report of a transmembrane c-kit mutation associated with human disease as well as the first identification of an activating c-kit mutation amenable to treatment with imatinib mesylate in systemic mastocytosis.
Materials and methods
Sequencing of c-kit
All individuals studied in this report provided informed consent on a protocol approved by the Institutional Review Board of the NIAID. gDNA and mRNA were isolated using DNeasy kit (Qiagen, Valencia, CA) and Trizol reagent (Invitrogen, Carlsbad, CA), according to the manufacturers' instructions. Total RNA was reverse transcribed to cDNA with Moloney murine leukemia virus reverse transcriptase without RNase H activity and oligo (dT) primer (Invitrogen). gDNA and cDNA were amplified by polymerase chain reaction (PCR) using oligonucleotide primers.5,6 Resulting PCR products were sequenced directly using the Big Dye Terminator kit (PE Biosystems, Foster City, CA) and analyzed on a capillary automated sequencer (ABI Prism 310 Genetic Analyzer; PE Biosystems).
Phe522Cys Kit plasmid construct
pcDNA3 plasmid vector coding for wild-type Kit was provided by Dr Gunnar Nilsson (University of Uppsala, Sweden). Site-directed mutagenesis was performed using the GeneTailor Site-Directed Mutagenesis System (Invitrogen) as directed by the manufacturer. Following the mutagenesis procedure, the resulting plasmid was sequenced to confirm the successful introduction of the mutation.
Transfection and Western blot analysis
Cos-7 cells (American Type Culture Collection, Rockville, MD) were grown in 6-well plates to 50% confluence. Overnight transfections using Phe522Cys Kit, wild-type Kit, and Asp816Val Kit plasmids were performed using the Fugene 6 System (Roche Molecular Biochemicals, Indianapolis, IN). Transfected cells were incubated with or without 100 ng/mL stem cell factor (SCF; Peprotech, Rocky Hill, NJ) for 8 hours at which point 1 μM imatinib mesylate (provided by Novartis Pharma, Basel Switzerland) was added to some wells for 2 hours. Cells were then lysed, the lysate placed on ice for 10 minutes, and clarified by centrifuging at 15 700g (13 000 rpm) at 4°C for 20 minutes. Equal aliquots of clarified cell lysate were heated at 100°C for 5 minutes in sodium dodecyl sulfate (SDS) buffer (80 mM Tris [tris(hydroxymethyl)aminomethane]–HCL pH 7.5, 2% SDS, 10% glycerol, 100 mM dithiothreitol, and 0.0005% bromphenol blue). Equal amounts of total protein were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose paper by electroblotting, and analyzed using horseradish peroxidase (HRP)–conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) against the primary antibody in conjunction with Western blot chemiluminescence (Pierce Chemical, Rockford, IL). The primary antibody, rabbit anti-Kit (C-19; Santa Cruz Biotechnology, Santa Cruz, CA) and the primary antibody, rabbit antiphosphoKit (Y719; Cell Signaling Technology, Beverly, MA) were purchased from the manufacturers.
Bone marrow mononuclear cell cultures
The bone marrow aspirate mononuclear cell fraction containing mast cells was separated using Histopaque (density = 1.077; Sigma, St Louis, MO) gradient centrifugation, contaminating red cells lysed in 0.8% ammonium chloride solution (StemCell Technologies, Vancouver, BC, Canada) for 10 minutes, and cultured at a concentration of 1 × 106 cells/mL in Stem-Pro serum-free medium (Life Technologies, Gaithersburg, MD) in the presence or absence of 100 ng/mL SCF (Peprotech) or 1 μM imatinib mesylate. Imatinib mesylate, provided by Novartis Pharma, was dissolved in water and kept at -20°C at a stock concentration of 10 mM.
Flow cytometric analysis of mast cells
Mast cells in bone marrow aspirates were identified by flow cytometry as a population highly expressing CD117 as described.7
Results
Identification of the transmembrane c-kit mutation
The most common molecular pathology in systemic mast cell disease is the Asp816Val c-kit mutation, which results in ligand-independent autophosphorylation of the Kit receptor protein. In our efforts to characterize additional mutations or polymorphisms in mast cell disease, we identified a patient who had a wild-type c-kit tyrosine kinase domain sequence. Sequencing of the entire c-kit cDNA in this patient led to the discovery of a novel heterozygous point mutation, at codon 522 within the transmembrane domain of Kit, resulting in replacement of the phenylalanine with cysteine (1586 T > G in cDNA sequence8 ; Figure 1A). The mutation was detectable in gDNA and cDNA in samples from peripheral blood, bone marrow, and oral epithelial cells. The mutation was not detectable in either parent of the patient, as well as in 69 unrelated healthy individuals and 22 patients with mastocytosis. These results suggest that the Phe522Cys c-kit mutation in this patient was a de novo germline mutation.
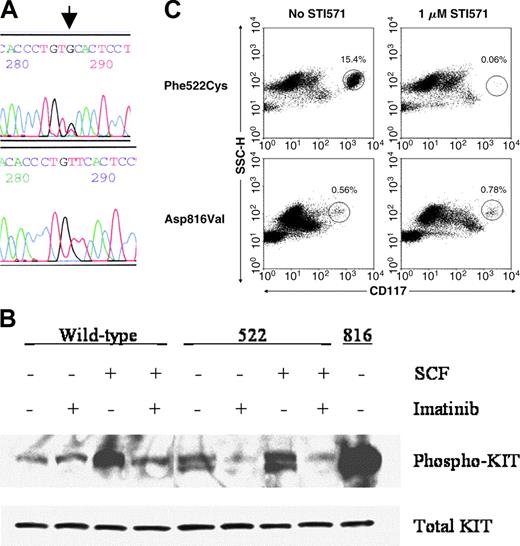
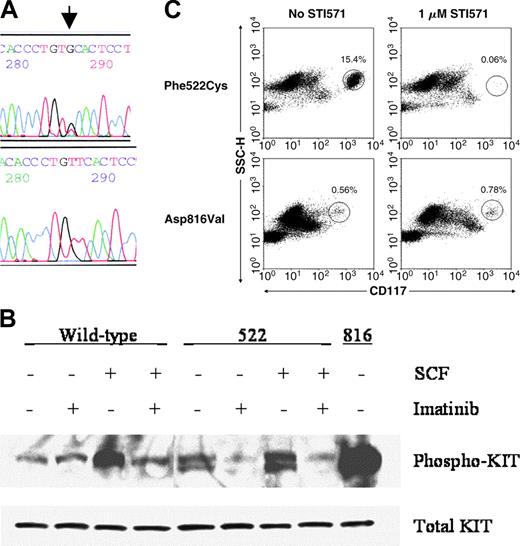
Demonstration of the Phe522Cys c-kit mutation and its functional analysis. (A) Direct sequencing of codon 522 of c-kit cDNA synthesized from bone marrow aspirate cells. Arrow denotes the T>G point mutation. Upper panel, patient; lower panel, healthy control. (B) Western blot analysis of phosphorylated Kit protein in Cos-7 cells transfected with wild-type, Phe522Cys, or Asp816Val mutants of Kit incubated with or without SCF and 1 μM imatinib mesylate. (C) In vitro sensitivity of the patient's bone marrow aspirate mast cells to 1 μM imatinib mesylate after 8 days of culture (top row) compared to those of a patient with a codon 816 c-kit mutation (bottom row). The circled populations represent mast cells, with their percentage denoted above the circle.
Demonstration of the Phe522Cys c-kit mutation and its functional analysis. (A) Direct sequencing of codon 522 of c-kit cDNA synthesized from bone marrow aspirate cells. Arrow denotes the T>G point mutation. Upper panel, patient; lower panel, healthy control. (B) Western blot analysis of phosphorylated Kit protein in Cos-7 cells transfected with wild-type, Phe522Cys, or Asp816Val mutants of Kit incubated with or without SCF and 1 μM imatinib mesylate. (C) In vitro sensitivity of the patient's bone marrow aspirate mast cells to 1 μM imatinib mesylate after 8 days of culture (top row) compared to those of a patient with a codon 816 c-kit mutation (bottom row). The circled populations represent mast cells, with their percentage denoted above the circle.
Functional analysis of the Phe522Cys c-kit mutation and in vitro response to imatinib mesylate
To assess the functional consequences of the presence of the Phe522Cys c-kit mutation, this point mutation was introduced by site-directed mutagenesis into a mammalian expression plasmid vector containing wild-type c-kit. Cos-7 cells were transfected with the plasmids containing either the wild-type or mutated c-kit and phosphorylation of Kit was assessed as a measure of activation. Wild-type Kit had a low level of autophosphorylation, which, as expected, increased after treatment with its ligand, SCF (Figure 1B). In contrast, Phe522Cys Kit had a high baseline autophosphorylation, which increased minimally after incubation with SCF. Autophosphorylation of both wild-type and Phe522Cys mutated Kit was inhibited effectively by 1 μM imatinib mesylate, a tyrosine kinase inhibitor with specificities against Kit, platelet-derived growth factor (PDGF) receptor, and bcr-abl proteins.9 Mast cells in the patient's bone marrow aspirate were almost completely eliminated when cultured with 1 μM imatinib mesylate for 8 days (Figure 1C).
Clinicopathologic features and response to imatinib mesylate therapy
The patient is a white woman who presented to the NIH Clinical Center at the age of 25 for evaluation of systemic mastocytosis. She had a history of cutaneous mastocytosis, diagnosed as urticaria pigmentosa when she was 5 months of age and which resolved by adolescence. Over the 3 years preceding the evaluation at NIH, the patient noted gradually worsening fatigue, musculoskeletal pain, episodic fevers, flushing and lightheadedness, and left upper quadrant pain. She was also noted to have a worsening pancytopenia with a white blood cell count of 3.5 × 109/L (3500/mm3), a hemoglobin level of 115 g/L (11.5 g/dL), and a platelet count of 76 × 109/L (76 000/mm3) in October 2000. Physical examination and diagnostic imaging of the abdomen elsewhere at that time revealed a massively enlarged spleen with mild hepatomegaly. Examination of the bone marrow revealed sheets and aggregates of mast cells in a perivascular, paratrabecular, and interstitial distribution, consistent with the diagnosis of systemic mast cell disease. One month later, she underwent splenectomy. Histopathologic examination of the spleen tissue revealed involvement with mast cell disease. The patient was started on interferon α (IFN-α) at 3 million units daily, approximately 5 weeks after splenectomy.
The patient was admitted to the NIH Clinical Center in June 2001, 5 months after the initiation of IFN-α. She was complaining of frequent (up to 10 times daily) episodes of lightheadedness associated with flushing, increased body temperature up to 38.3°C (101°F), diffuse musculoskeletal pain that necessitated use of opioid analgesics, headaches, night sweats, and fatigue. Physical examination was unremarkable except for a mildly flushed skin. The white blood cell count was 4.86 × 109/L (4860 mm3), hemoglobin level 125 g/L (12.5 g/dL), and platelet count 318 × 109/L (318 000 mm3). Serum tryptase level was markedly elevated at 173 ng/mL (normal < 11.5 ng/mL). A bone scan with 99Tc methylene diphosphonate showed diffusely increased uptake of tracer throughout the skeleton, and in particular major joint regions such as shoulders, hips, and knees. A plain x-ray of the knees and a bone densitometry study, however, did not demonstrate any abnormalities.
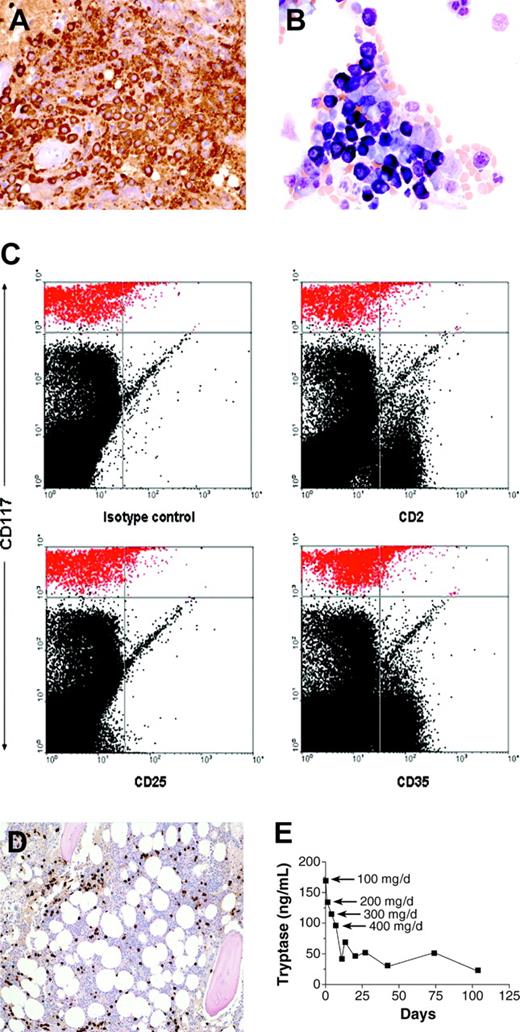
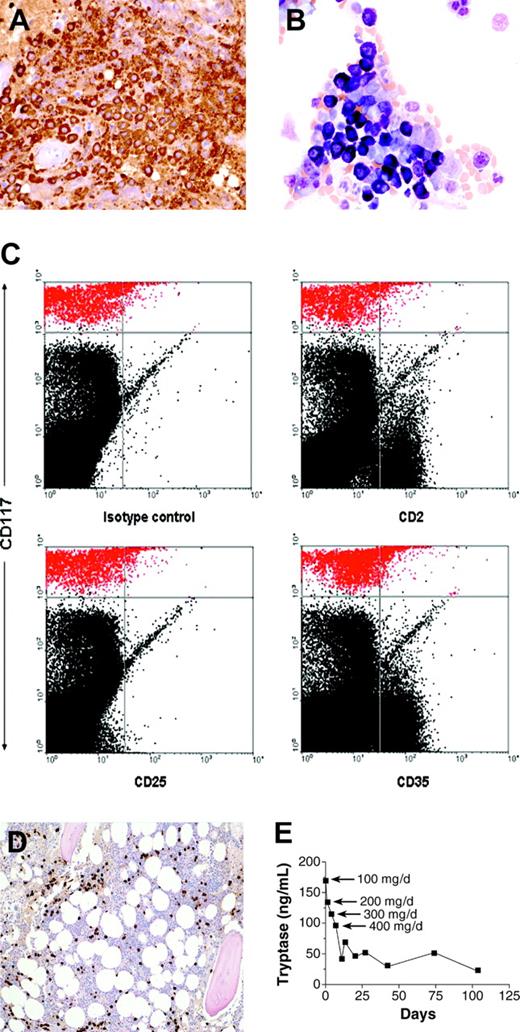
Her bone marrow biopsy revealed approximately 50% of the marrow to be comprised of mast cells. These mast cells were round, highly granulated, and contained a centrally located nucleus (Figure 2A-B). Flow cytometric analysis of the bone marrow aspirate mast cells revealed absence of surface expression of CD2, CD25, and CD35 (Figure 2C). These results are in contrast to the patients with typical systemic mastocytosis, whose mast cells have abnormal morphology with spindle shapes, hypogranulated cytoplasm, eccentric nucleus, and cytoplasmic projections, in addition to aberrant expression of surface marker proteins including CD2, CD25, and CD35.7,10 Other markers reported to be aberrantly expressed in systemic mastocytosis (CD63, CD69, and CD88)11,12 were not analyzed in this study.
Histopathologic features of bone marrow biopsy and aspirate and response to therapy. (A) Bone marrow biopsy section immunohistochemically stained for tryptase (brown color); (B) aspirate stained with Wright-Giemsa; (C) flow cytometric analysis of the bone marrow aspirate. Mast cells are depicted in red in the upper part of the dot plots. (D) Bone marrow biopsy obtained 2 months after initiation of therapy, stained for tryptase. Compare with panel A. (E) Serum tryptase level during the course of treatment. Imatinib mesylate was started at a dosage of 100 mg once daily and increased to 200, 300, and 400 mg daily on days 5, 8, and 12, respectively. Original magnification, × 40 (A-B); and × 10 (D).
Histopathologic features of bone marrow biopsy and aspirate and response to therapy. (A) Bone marrow biopsy section immunohistochemically stained for tryptase (brown color); (B) aspirate stained with Wright-Giemsa; (C) flow cytometric analysis of the bone marrow aspirate. Mast cells are depicted in red in the upper part of the dot plots. (D) Bone marrow biopsy obtained 2 months after initiation of therapy, stained for tryptase. Compare with panel A. (E) Serum tryptase level during the course of treatment. Imatinib mesylate was started at a dosage of 100 mg once daily and increased to 200, 300, and 400 mg daily on days 5, 8, and 12, respectively. Original magnification, × 40 (A-B); and × 10 (D).
IFN-α was discontinued when no clinical or histopathologic evidence of improvement was noted after 8 months on this drug. The patient was treated symptomatically for the next 19 months with no improvement in clinical status. Because the results obtained from in vitro studies suggested that imatinib mesylate would be an effective therapy for this patient, a clinical protocol was initiated in which the patient was given increasing doses of imatinib mesylate until a daily dose of 400 mg was reached. The patient experienced headaches, nausea, and vomiting at the dosage of 400 mg/d, which gradually resolved after several weeks of treatment at this dosage.
A bone marrow biopsy and aspirate, performed after 2 months of therapy with imatinib mesylate, revealed a notable reduction in the extent of bone marrow mast cell infiltration with mast cells representing less than 10% of the bone marrow biopsy (Figure 2D) and approximately 2% of the aspirate. Serum tryptase levels decreased to 115 ng/mL after 4 doses of 100 mg/d imatinib mesylate, and to 21.5 ng/mL after approximately 3.5 months on 400 mg/d (Figure 2E), and remain at 20 ng/mL as of the last measurement at 7 months on treatment. The reduction in mast cell numbers in bone marrow and serum tryptase was accompanied by a dramatic improvement in the patient's symptoms. Five months after the initiation of imatinib mesylate therapy, the patient reported resolution of episodes of lightheadedness, improvement in energy level, and a reduction of musculoskeletal pain with discontinuation of narcotic analgesics and as-needed use of H1 antihistamines.
Discussion
c-kit is a transmembrane receptor protein with intrinsic tyrosine kinase activity. Gain-of-function mutations rendering Kit independent of its ligand, SCF, have been described in gastrointestinal stromal tumors (GISTs),13 acute myeloid leukemias,14 and germ cell tumors15 in addition to systemic mastocytosis. The location of the mutation in Kit carries important pharmacogenomic implications for the treatment of these disorders; the majority of the GISTs are associated with mutations in the juxtamembrane domain (the intracellular segment between the transmembrane and tyrosine kinase domains), whereas the other conditions are associated with mutations in the enzymatic domain of the molecule. The presence of an enzymatic site mutation, such as the codon 816 mutations encountered in systemic mastocytosis, renders Kit resistant to inhibition by imatinib mesylate, whereas Kit with a juxtamembrane mutation remains sensitive to imatinib mesylate.
These observations suggest that mutations in distinct c-kit domains appear to be associated with different forms of human disease. Thus, although juxtamembrane and tyrosine kinase domains appear to be important in transformation of gastrointestinal pacemaker cells and mast cells, respectively, the function of the transmembrane domain had remained unclear. Our report is the first description of a transmembrane c-kit mutation in human disease and highlights the importance of this region in mast cell growth. This is because the presence of this germline mutation did not cause any identifiable pathology other than mast cell disease in this patient. The observation that the presence of the Phe522Cys mutation increased the autophosphorylation of Kit provides evidence to support a regulatory role of the transmembrane region in functioning of the enzymatic domain of the molecule. This conclusion is also consistent with the proposed mechanism of action of imatinib mesylate, in which the drug is able to bind and inhibit Kit as long as the structure of the enzymatic pocket remains unaltered.16
The Phe522Cys c-kit mutation is further associated with an unusual variant of mast cell disease of a well-differentiated phenotype. This unique phenotype of mast cells does not appear to be a result of IFN-α therapy because examination of the bone marrow aspirate prior to the institution of IFN-α revealed the same morphologic features of mast cells. The observations that Kit bearing the Phe522Cys transmembrane mutation had higher levels of autophosphorylation and its inhibition with imatinib mesylate resulted in regression of disease provide a strong argument about a causative role for the Phe522Cys mutation in development of this disease variant. It is, however, not known whether all patients with well-differentiated mastocytosis have a transmembrane activating c-kit mutation. This is in part due to the rarity of the disease; a review of bone marrow pathology, flow cytometric data, and tryptase levels from 68 consecutive patients with mastocytosis evaluated in our clinic revealed the patient in this report to be the only patient with well-differentiated mast cell phenotype and yet met the diagnostic criteria for systemic mastocytosis.10,17 A multicenter study is thus needed to assess the true frequency of this disease variant and whether it is associated with Phe522Cys or other activating c-kit mutations.
It should be emphasized that most patients with systemic mastocytosis evaluated in our clinic have aberrant mast cell morphology, carry codon 816 c-kit mutations, and thus are not candidates for imatinib mesylate therapy. Recently, however, imatinib mesylate has been reported to result in histologic or clinical responses in 5 of 12 patients with mastocytosis.18 Although these patients were reported to lack a codon 816 or juxtamembrane mutations, it is not clear whether they had any distinguishing histopathologic changes in their bone marrow mast cells or carried other c-kit mutations. Together, these observations underscore the necessity of a mutational analysis of Kit including codon 816, before contemplating therapy with imatinib mesylate in patients with mastocytosis. Careful examination of the mast cell morphology of the bone marrow may provide clues to the identification of the subtype of mastocytosis herein reported.
Supported by intramural funds from National Institute of Allergy and Infectious Diseases (NIAID), National Cancer Institute (NCI), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-11-3816.