Abstract
Human CD99 is a small highly O-glycosylated cell-surface protein expressed on most leukocytes. It was recently found to be expressed at endothelial cell contacts and to participate in the transendothelial migration (TEM) of monocytes in vitro. In order to analyze the physiologic relevance of CD99 in vivo we searched for the mouse homolog. We cloned a mouse cDNA coding for a protein 45% identical in its sequence with human CD99. Based on the cDNA, we generated antibodies against this mouse homolog of CD99, which detected the antigen on most leukocytes, on endothelia of various tissues, and at cell contacts of cultured endothelial cells. Cell aggregation of CD99-transfected Chinese hamster ovary (CHO) cells was completely blocked by anti-CD99 antibodies. The same antibodies inhibited TEM of lymphocytes in vitro, independent of whether T cells or endothelial cells were preincubated with antibodies. In a cutaneous delayed-type hypersensitivity (DTH) reaction, anti-CD99 antibodies inhibited the recruitment of in vivo–activated T cells into inflamed skin as well as edema formation. We conclude that mouse CD99 participates in the TEM of lymphocytes and in their recruitment to inflamed skin in vivo. This establishes CD99 as a valid target for interference with cutaneous inflammatory processes.
Introduction
Recruitment of leukocytes into inflamed tissue requires emigration from the bloodstream and migration of the leukocytes through the endothelial lining and the basement membrane of the blood vessels. The whole process is regulated by a cascade of molecular interactions,1,2 which is initiated by the selectins mediating leukocyte capturing, followed by the chemokines that activate leukocyte integrins and stimulate extravasation.3-5
The molecules initiating leukocyte extravasation by controlling docking and migration on the endothelial cell surface have been well studied to date. In contrast, much less is known about the process of transmigration through the blood vessel wall (diapedesis).6 Only very few endothelial membrane proteins are known to be involved. Platelet-endothelial cell adhesion molecule-1 (PECAM-1) was the first to be identified in this context. It can support cell adhesion by homophilic interactions and it is able to transmit signaling.7 In numerous reports, antibodies against PECAM-1 as well as PECAM-Fc fusion proteins were demonstrated to inhibit monocyte and neutrophil, but not lymphocyte, migration through endothelial cell monolayers in vitro as well as neutrophil extravasation into inflammatory sites in vivo.8-10
The junctional adhesion molecule (JAM or JAM-A) is enriched at endothelial tight junctions and was shown to be involved in transendothelial migration (TEM) of monocytes and neutrophils.11,12 JAM-A forms a protein family with JAM-B and JAM-C, which may have related functions.9,13-17
CD99 is the third type of endothelial membrane protein that was recently reported to participate in the transmigration of monocytes through human umbilical vein endothelial cells (HUVECs) in vitro.18 A monoclonal antibody (mAb) raised against endothelial cells that stained endothelial cell contacts and inhibited TEM turned out to react with CD99, a long-known leukocyte membrane protein that had not yet been described on endothelial cells and had never been considered to be involved in this process. CD99 is expressed on most leukocytes where its function is not well understood, although several effects can be triggered by cross-linking the molecule with primary and secondary antibodies. CD99 has been described as a costimulatory molecule on T cells19,20 and a target for antibody-induced integrin-independent aggregation of thymocytes,21 β2-integrin–dependent aggregation of a B lymphoblastoid cell line,22 α4β1-integrin–mediated adhesion of T cells to endothelial vascular cell-adhesion molecule 1 (VCAM-1),23 and apoptosis.24,25 However, antibody stimulation of many surface proteins on lymphocytes led to similar effects and in several cases more effectively than described for CD99.
Human CD99 is a highly O-glycosylated, small protein of only 32 kDa26 with a unique structure without resemblance to any known protein family. The gene coding for the CD99 protein27 is located in the pseudoautosomal region of the human X and Y chromosomes.28 Other related human proteins are the erythrocyte blood group antigen Xga,29 and the CD99-like gene product (CD99L2),30 for which orthologs in mice and humans have been described.30 Cross-hybridization experiments of CD99 showed that its nucleotide conservation is restricted to other primate homologs and no homologous genes were detectable in this way in nonprimate species.31
In order to analyze the physiologic relevance of CD99 in vivo we searched for the mouse homolog. Here we report on the identification and cloning of a mouse gene coding for a protein 45% identical in its sequence with human CD99, which we found to represent the mouse CD99 homolog. Based on the cDNA, we generated antibodies against the mouse homolog of CD99, which we have used here to analyze its function. We demonstrate that it can support homotypic cell adhesion, and that adhesion blocking antibodies against it inhibit TEM of lymphocytes in vitro as well as the recruitment of T cells into inflamed skin in vivo. This protein is likely to be the homolog and functional analog of human CD99 in the mouse. This is the first demonstration of the participation of this membrane protein in the process of TEM of lymphocytes in vitro and lymphocyte recruitment into inflamed tissue in vivo.
Materials and methods
Cell culture
The following cells were propagated as described: proteinlipid protein (PLP)–specific T helper 1 (TH1) memory/effector T-cell lines SJL.PLP3 and SJL.PLP7,32 Chinese hamster ovary (CHO) cells,33 and bEnd.5 mouse endothelioma cells.34 CHO cell lines expressing mouse CD99-Fc or mouse CD99 were generated by electroporation according to established procedures.35
Antibodies
Polyclonal rabbit antisera against mouse CD99 were generated against CD99-Fc fusion protein. For purification of polyclonal antibodies, antibodies against the immunoglobulin G1 (IgG1)–Fc part were removed by incubation with human IgG1 coupled to cyanogene bromide (CNBr)–activated Sepharose (Amersham Pharmacia Biotech, Freiburg, Germany); thereafter mouse CD99-specific antibodies were affinity-purified on mouse CD99-Fc immobilized on CNBr-Sepharose. F(ab′)2 and Fab fragments were generated with immobilized pepsin and papain on beads (Pierce, Rockford, IL), respectively, according to the manufacturer's protocol. Uncleaved IgG and Fc fragments were removed using protein A sepharose (Amersham Pharmacia Biotech). Purity and proper size of the IgG, F(ab′)2, and Fab fragments were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Relative binding efficiency was tested by enzyme-linked immunosorbent assay (ELISA) using CD99-Fc as the antigen. No endotoxin was detectable. Affinity-purified polyclonal rabbit antibodies against mouse endothelial cell-selective adhesion molecule (ESAM) were generated as described previously.35 The following monoclonal antibodies were purified from hybridoma cells: anti–mouse intercellular adhesion molecule-1 (ICAM-1, YN1/1.7, rat IgG-2b, hybridoma obtained from American Type Culture Collection [Manassas, VA])36 ; anti–mouse vascular endothelial (VE)–cadherin (11D4.1, rat IgG-2a)37 ; anti–mouse E-selectin (UZ4, rat IgM),38 anti–mouse P-selectin (RB40.34, rat IgG1),39 and anti–mouse β1-integrin chain (cross-reactive with hamster antigen) (9EG7).40 The following commercially available antibodies were used: fluorescein isothiocyanate (FITC)–labeled antibodies to mouse CD19, CD3ϵ, CD4, CD8, Gr1, and CD11c (BD Pharmingen, San Diego, CA), and anti-7/4 antigen (Serotec, Raleigh, NC). The following antibodies were also used: biotinylated anti–rabbit IgG, cyanin 3 (Cy3)–conjugated anti–rabbit and anti–rat IgG, FITC-conjugated anti–rabbit IgG, phycoerythrin-conjugated anti–rabbit IgG, and peroxidase-conjugated anti–rabbit IgG (Dianova, Hamburg, Germany); and FITC-labeled and peroxidase-labeled polyclonal antibodies to rabbit IgG F(ab′)2 (Acris, Hiddenhausen, Germany). Immunofluorescence staining and Western blots were done as described.35
Cloning of mouse CD99
For generation of a mouse CD99-Fc fusion protein, total cellular RNA was isolated from mouse spleen cells using the Trizol reagent (Invitrogen, Frederick, MD) according to the manufacturer's protocol. Based on reverse transcriptase–polymerase chain reaction (RT-PCR), a cDNA fragment coding for the extracellular part of mouse CD99 (based on GenBankTM/EBI Data Bank accession numbers BC019482) was generated from total spleen RNA, covering amino acid residues 1 to 138 using a HindIII site containing sense oligonucleotide 5′-TAGTAGAAGCTTTCATCACGGCCCG GCCATGG-3′ and an EcoRI site containing antisense oligonucleotide 5′-CTACTAGAATTCACTTACCTAAGCCCTGGGGCGTCC CTTCC-3′. The product was inserted into a pcDNA3-based Fc-construct vector (pcDNA3/hIgG-Fc) in frame and upstream of a fragment of human IgG1 covering bases 553 to 1803 (hinge, CH2, CH3). A cDNA covering the protein coding sequence of mouse CD99 was generated by PCR from the cDNA clone BF538213 obtained from the IMAGE Consortium (Lawrence Livermore National Laboratories, Livermore, CA) via the RZPD (German Resource Center for Genome Research, Heidelberg, Germany) using the sense oligonucleotide 5′-TCATCACGGCCCGGCCATGGC-3′ and the antisense oligonucleotide 5′-GCCATGGCGTCATCTACACG-3′. The PCR product was inserted into a pcDNA3-based vector (Invitrogen). The respective amino acid sequence was analyzed for the topology of putative signal sequence and transmembrane region by the SMART.EMBL program (European Molecular Biology Laboratory, Heidelberg, Germany).41
Immunohistochemistry and flow cytometry
Animals were anesthetized using Isoflurene anesthesia (Abbott, Wiesbaden, Germany) and were perfused with 10 mL phosphate-buffered saline (PBS) through the left ventricle of the heart. Tissue was removed, embedded in Tissue-tec (OCT; Miles, Vogel, Giessen, Germany), and snap-frozen. Cryostat sections (6 μm) were stained as described.42 To detect accessibility of luminal antigens in blood vessels, mice were injected intravenously with 100 μg primary antibody, anesthetized 15 minutes later, and perfused first with PBS to remove unbound antibody and then with 1% paraformaldehyde (PFA) to fix bound antibody. Immunohistology was performed omitting the first antibody. Cryostat sections and immunofluorescence staining were imaged using a Zeiss Axioskop 2 upright microscope (Zeiss, Jena, Germany) equipped with Zeiss Plan-Apochromat 20×/0.6 objective lenses and a SPOT 2.2.1. camera (Visitron Systems, Puchheim, Germany).All images were captured using SPOT advanced RT 3.0 software (Diagnostic Instruments, Puchheim, Germany). Flow cytometry was done as described.43 CD11c+ cells were isolated from spleen and enriched for CD11c+ cells as described.44
Aggregation assay
Our assay was modified from a previously described method.45 Cells were rinsed twice with PBS, detached in PBS containing 5 mM EDTA (ethylenediaminetetraacetic acid), washed, and resuspended in aggregation buffer (Hanks buffered saline solution 14170 [HBSS-14170; Gibco, Grand Island, NY] plus 2 mM CaCl2, 1 mM MgSO4, 25 mM Hepes [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4). Then, 200 μL cell suspension (2 × 106 cells/mL) was preincubated for 30 minutes at 4°C with 30 μg/mL antibodies for 30 minutes at 4°C. Warm aggregation buffer (1800 μL) was added and cells were allowed to aggregate for 60 minutes at 37°C in a rotating shaker (60 rpm). The reaction was stopped by the addition of 0.5 mL 25% glutaraldehyde. Aggregation was quantified by counting representative aliquots from each sample using a cell counter (CASY; Schärfe-System, Reutlingen, Germany). Quantification of aggregation was estimated by the following formula: % aggregation = (N0 – Nt)/N0 × 100, where Nt is the total number of particles at the incubation time (t), and N0 is the total number of cells.
Adhesion assay
bEnd.5 cells (2.2 × 104) were cultured per well of 16-well glass chamber slides (Nunc, Wiesbaden, Germany) coated with 50 μg/mL fibronectin (Boehringer Mannheim, Mannheim, Germany) for 2 days. Endothelioma cells were stimulated with 5 nM human recombinant tumor necrosis factor α (TNF-α) 16 hours before the experiment. At 30 minutes prior to the assay, cells were incubated with 30 μg/mL Ab in adhesion medium (Dulbecco modified Eagle medium, 25 mM Hepes, 5% fetal calf serum) at room temperature. After removal of Abs, 1 × 105 lymphocytes were added in 100 μL adhesion medium for 30 minutes on a rocking platform (Fischer, Frankfurt/Main, Germany). Assays were washed twice in PBS and fixed in 2.5% glutaraldehyde in PBS at 4°C for 2 hours. Assays were analyzed by video associated light microscopy (NIH Image software; National Institutes of Health [NIH], Rockville, MD).
Transmigration assay
TEM of lymphocytes was analyzed as previously described,34 with 1 × 105 T cells added to 5 × 104 bEnd.5 cells that had been grown for 2 days in 6.5-mm Transwells (Costar, Bodenheim, Germany) with 5-μm pore size, coated with 50 μg/mL laminin (Boehringer Mannheim). The last 16 hours before adding lymphocytes, bEnd.5 cells were stimulated with 5 nM human recombinant TNF-α. Assays were run at 37°C and 10% CO2 for 2 hours in the absence of chemokines or for 30 minutes, if 100 ng/mL stromal-derived factor 1 (SDF-1) was added to the bottom chamber.
Contact hypersensitivity and T-cell immigration into inflamed skin
Female Balb/c mice, 6 to 10 weeks of age, were obtained from Harlan-Winkelmann (Borchen, Germany). A cutaneous delayed-type hypersensitivity (DTH) reaction was induced by skin painting with 20 μL 0.5% 2,4-dinitrofluorobenzene (DNFB) in acetone–olive oil (4:1) on day –21 and –20 on the shaved abdomen and rechallenged on day 4 on both shaved flanks for isolation of T cells or on day –1 with 12 μL 0.5% DNFB on the front and dorsal surface of the right ear. For isolation of T cells, axilar and inguinal lymph nodes were prepared and cells were collected in HBSS. CD19+ cells were depleted using rat α-mouse CD19 bound to sheep α-rat IgG bearing Dynabeads M-450 (Dynal Biotech, Hamburg, Germany). All remaining cells stained positive for CD3ϵ, among them 60% to 70% were CD4+ T cells. After B-cell depletion, the cell suspension contained less than 3% dead cells. T cells were labeled with 20 μCi/mL (750 kBq/mL) sodium [51Cr]chromate for 1 hour at 37°C, and 2 × 106 cells were injected with 70 μg antibody in 250 μL PBS into the tail vein of each mouse. After 15 hours, mice were killed and the radioactivity in the inflamed right and noninflamed left ear, as well as in various other organs (spleen, liver, lung), was measured. Statistical analysis was done by Student t test.
Antibody effects on edema formation were tested by sensitizing C57/Bl6 mice at day –5 by skin painting with 75 μL 0.5% DNFB in aceton–olive oil (4.1) solution on the shaved abdomen, and by challenging the ears on day 0 the front and dorsal surface of the right ear with 12 μL 0.5% DNFB. Immediately prior to the challenge of the ear skin, inhibitory antibodies were injected intravenously and ear swelling was measured with a spring-loaded micrometer (Mitutoyo, Kawasaki, Japan) 10 hours after challenge. Swelling was determined as the thickness of the DNFB-challenged ear compared with the thickness of the untreated ear in sensitized mice and was expressed in micrometers. Each group consisted of at least 5 mice. Total peripheral leukocyte counts were determined after erythrocyte lysis.
Results
Cloning of a mouse homolog for human CD99
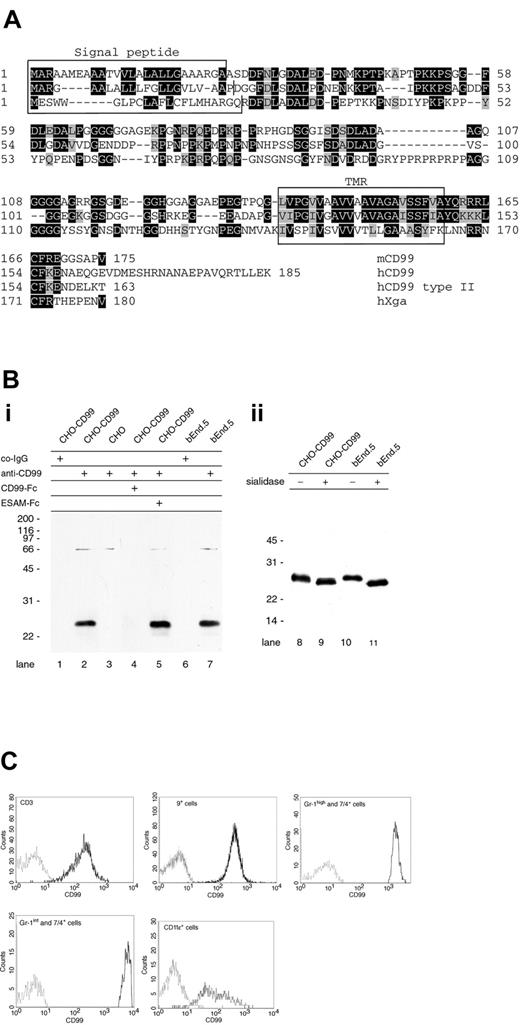
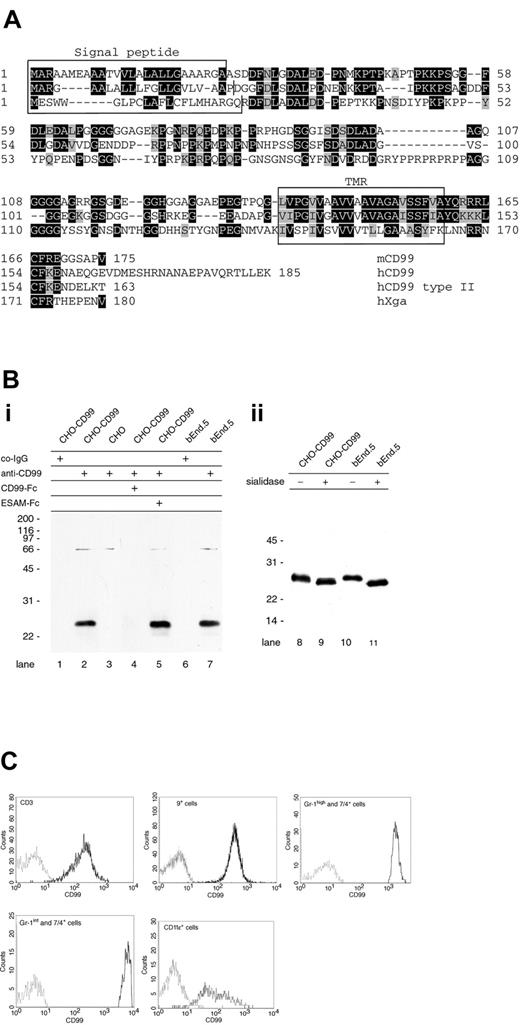
Searching the gene databases with the nucleotide sequence of human CD99 did not reveal homologous sequences in the mouse. A search with the protein sequence of human CD99 for translated nucleotide sequences revealed a mouse clone (AK004342) that was at that time tentatively described in the National Center for Biotechnology Information (NCBI, Rockville, MD) database as homologous to human CD99. Since only a part of the sequence coded for an open reading frame, we searched for further mouse cDNAs related to this clone and found clone BC019482 that contained an open reading frame coding for a 175–amino acid protein. Sequencing the IMAGE Consortium cDNA BF538213 revealed full match with BC019482 despite 4 mismatches in the database entry. The sequence was further verified by comparison with cDNAs obtained by RT-PCR from polyA+-RNA isolated from mouse spleen and from mouse bEnd.5 endothelioma cells. Human CD99 exists as 2 splice variants of 185 and 163 amino acids in length varying in their C-terminal end. The isolated mouse cDNA coded for a type I membrane protein; its mature form contains 149 amino acids (calculated molecular weight, 17.008 kDa) with 45% identical amino acid sequence compared with the shorter of the 2 splice variants of human CD99 (Figure 1A). Similar to human CD99, the protein is rich in glycine and proline residues, and has no N-glycosylation sites and no characteristic domains or motifs. No genomic sequences or splice variants for mouse CD99 were listed in public gene databases. Since the human and mouse orthologs of CD99L2 have been identified,30 the Xga blood group protein is the only other known candidate that could represent an ortholog for the mouse protein we identified. As shown in the alignment in Figure 1A, the transmembrane domains of human and mouse CD99 show 68% identity and 91% homology (conservative exchanges), whereas the transmembrane domain of the Xga protein is only 32% identical and 54% homolog. Based on the sequence similarities and the results obtained from studying the corresponding mouse protein, we conclude that we have cloned a mouse equivalent of human CD99. During processing of this manuscript, cloning of the same protein has been reported.46
Cloning and expression of CD99. (A) Deduced amino acid sequence of mouse CD99 (top sequence) and alignment with human CD99 (2 middle sequences) and human Xga protein (bottom sequence). Both splice variants of human CD99 (hCD99 type I and type II) are depicted and vary only at their C-terminus. Except for the cleavage site of the signal sequence of human CD99, which is based on sequencing of the mature CD99 protein,27 the putative signal sequences and the transmembrane regions (marked in boxes) were predicted by the SMART.EMBL program. ▪ indicates identical amino acids; ▪, homologous amino acids; and □, signal sequence (top panels) or transmembrane region (bottom panel). (Bi) Detection of mouse CD99 in Western blots. Cell lysates of CD99-transfected CHO cells (lanes 1-2, 4-5), mock-transfected CHO cells (lane 3), and mouse bEnd.5 endothelioma cells (lanes 6-7) were analyzed in immunoblots for reactivity with preimmune serum IgG (co-IgG: lanes 1, 6) or affinity-purified polyclonal antibodies against CD99 (anti-CD99: lanes 2-5, 7). To further control specificity, antibodies were mixed with either recombinant CD99-Fc (lane 4) or with ESAM-Fc (lane 5). (Bii) Lysates of CHO-CD99 cells or bEnd.5 cells were either mock incubated (–) or incubated with 5 mU sialidase from Arthrobacter ureafaciens (+) for 6 hours at 37°C prior to immunoblotting. Binding of first antibody was detected by incubating with peroxidase-conjugated secondary antibodies followed by visualization via chemiluminescence. (C) Cell-surface expression of CD99 on T cells (CD3ϵ+), B cells (CD19+), granulocytes (Gr-1high and 7/4+), and monocytes (Gr-1int and 7/4+) from peripheral blood mouse leukocytes (PBML) and of CD11c+ cells from mouse spleen was determined by 3-color FACS analysis. Negative staining was determined with control IgG from preimmune serum (gray line) and CD99 staining, with affinity-purified anti-CD99 antibodies (black lines).
Cloning and expression of CD99. (A) Deduced amino acid sequence of mouse CD99 (top sequence) and alignment with human CD99 (2 middle sequences) and human Xga protein (bottom sequence). Both splice variants of human CD99 (hCD99 type I and type II) are depicted and vary only at their C-terminus. Except for the cleavage site of the signal sequence of human CD99, which is based on sequencing of the mature CD99 protein,27 the putative signal sequences and the transmembrane regions (marked in boxes) were predicted by the SMART.EMBL program. ▪ indicates identical amino acids; ▪, homologous amino acids; and □, signal sequence (top panels) or transmembrane region (bottom panel). (Bi) Detection of mouse CD99 in Western blots. Cell lysates of CD99-transfected CHO cells (lanes 1-2, 4-5), mock-transfected CHO cells (lane 3), and mouse bEnd.5 endothelioma cells (lanes 6-7) were analyzed in immunoblots for reactivity with preimmune serum IgG (co-IgG: lanes 1, 6) or affinity-purified polyclonal antibodies against CD99 (anti-CD99: lanes 2-5, 7). To further control specificity, antibodies were mixed with either recombinant CD99-Fc (lane 4) or with ESAM-Fc (lane 5). (Bii) Lysates of CHO-CD99 cells or bEnd.5 cells were either mock incubated (–) or incubated with 5 mU sialidase from Arthrobacter ureafaciens (+) for 6 hours at 37°C prior to immunoblotting. Binding of first antibody was detected by incubating with peroxidase-conjugated secondary antibodies followed by visualization via chemiluminescence. (C) Cell-surface expression of CD99 on T cells (CD3ϵ+), B cells (CD19+), granulocytes (Gr-1high and 7/4+), and monocytes (Gr-1int and 7/4+) from peripheral blood mouse leukocytes (PBML) and of CD11c+ cells from mouse spleen was determined by 3-color FACS analysis. Negative staining was determined with control IgG from preimmune serum (gray line) and CD99 staining, with affinity-purified anti-CD99 antibodies (black lines).
Mouse CD99 is expressed on leukocytes and at endothelial cell contacts
We generated rabbit antibodies against a mouse CD99-Fc fusion protein containing the complete extracellular part of CD99 fused to the Fc part of human IgG1. Affinity-purified anti-CD99 antibodies reacted specifically in immunoblots with a 25-kDa protein in mouse CD99-transfected CHO cells but not in mock-transfected CHO cells (Figure 1B). A protein of similar molecular weight was recognized in bEnd.5 cells. No signal was obtained if antibodies were preincubated with CD99-Fc, whereas preincubation with another Fc-fusion protein (ESAM-Fc) did not interfere with the blot signal. The weak signal at 66 kDa is due to some reactivity against bovine serum albumin (BSA) since preincubation of the antibodies with BSA suppressed the signal (not shown). As described for human CD99, we could demonstrate that mouse CD99 carries sialic acid, arguing for O-glycosylation since no N-glycosylation sites are present (Figure 1B).
Similar to human CD99 that is found on most leukocytes, the mouse counterpart was expressed on peripheral blood T and B cells, granulocytes, monocytes, and CD11c+ cells as analyzed by flow cytometry (Figure 1C).
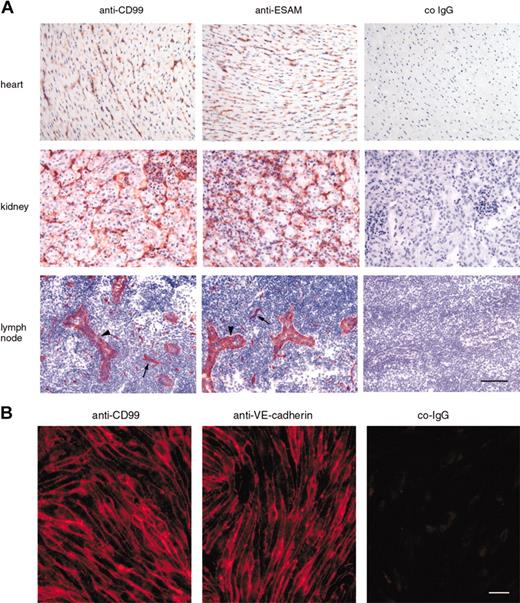
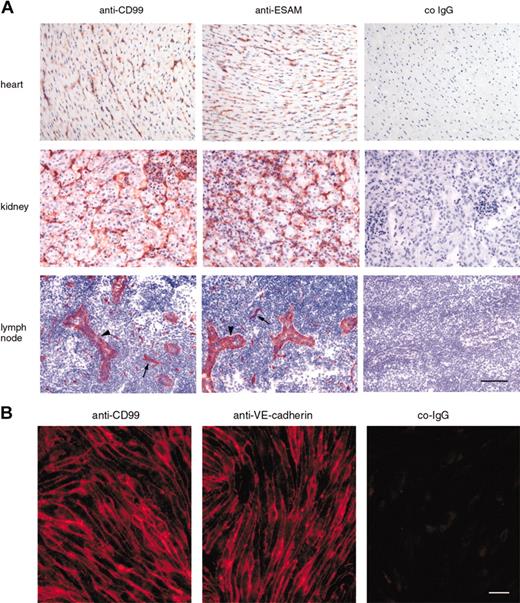
Antibodies against mouse CD99 also recognized endothelial cells in various tissues such as the heart, kidney, and lymph nodes (Figure 2A). In order to analyze endothelial staining in lymph nodes independent of the expected staining of lymphocytes, anti-CD99 antibodies were intravenously injected into mice for 15 minutes, before perfusion of the animal with PBS. Lymph node sections were then stained, omitting the first antibody to visualize the anti-CD99 antibodies that had previously bound in vivo. In this way, endothelium of capillaries as well as of high endothelial venules were strongly stained (Figure 2A). On cultured bEnd.5 cells, CD99 was brightly stained at intercellular contacts (Figure 2B). Thus, mouse CD99 is found on vascular endothelium and is concentrated at interendothelial cell contacts.
Expression of mouse CD99 on endothelial cells. (A) Immunoperoxidase staining of cryostat sections of mouse heart, kidney, and peripheral lymph nodes with affinity-purified antibodies against mouse CD99, mouse ESAM, and control rabbit IgG (co-IgG) from preimmune serum (as indicated). Sections of heart and kidney were incubated with first antibodies, followed by washing and incubation with secondary and tertiary reagent. In order to avoid lymphocyte staining in the lymph nodes, mice were injected intravenously with first antibody, anesthetized 15 minutes later, and perfused with PBS to remove unbound antibody and then with PFA to fix bound antibody. Cryostat sections of lymph nodes were then incubated with only secondary and tertiary reagent. Arrowheads point to high endothelial venules (HEVs), and arrows to capillaries. Bar = 70 μm. (B) Immunofluorescence staining of mouse bEnd.5 endothelioma cells with affinity-purified antibodies against CD99, mAb against VE-cadherin, and negative control IgG from the respective preimmune serum (as indicated). First antibodies were detected with Cy3-conjugated secondary antibodies and visualized by fluorescence microscopy. Bar = 20 μm.
Expression of mouse CD99 on endothelial cells. (A) Immunoperoxidase staining of cryostat sections of mouse heart, kidney, and peripheral lymph nodes with affinity-purified antibodies against mouse CD99, mouse ESAM, and control rabbit IgG (co-IgG) from preimmune serum (as indicated). Sections of heart and kidney were incubated with first antibodies, followed by washing and incubation with secondary and tertiary reagent. In order to avoid lymphocyte staining in the lymph nodes, mice were injected intravenously with first antibody, anesthetized 15 minutes later, and perfused with PBS to remove unbound antibody and then with PFA to fix bound antibody. Cryostat sections of lymph nodes were then incubated with only secondary and tertiary reagent. Arrowheads point to high endothelial venules (HEVs), and arrows to capillaries. Bar = 70 μm. (B) Immunofluorescence staining of mouse bEnd.5 endothelioma cells with affinity-purified antibodies against CD99, mAb against VE-cadherin, and negative control IgG from the respective preimmune serum (as indicated). First antibodies were detected with Cy3-conjugated secondary antibodies and visualized by fluorescence microscopy. Bar = 20 μm.
Mouse CD99 supports homotypic cell adhesion
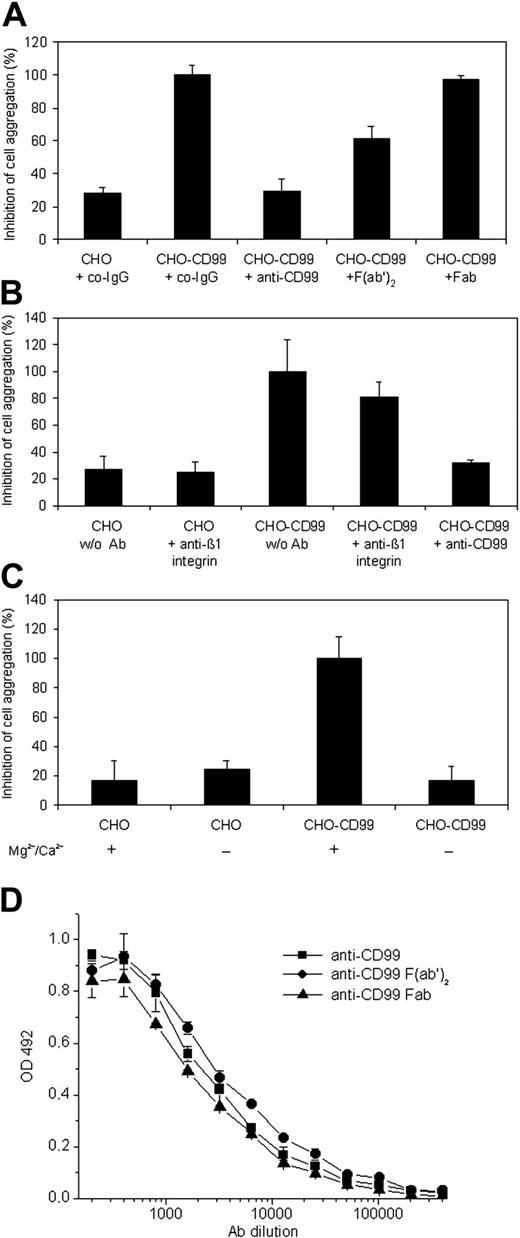
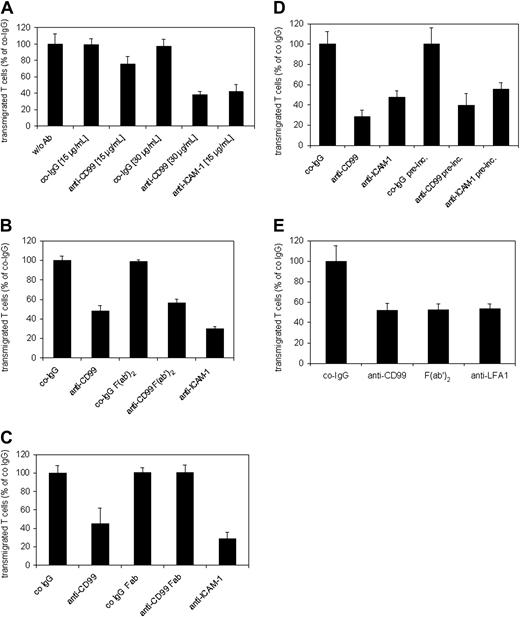
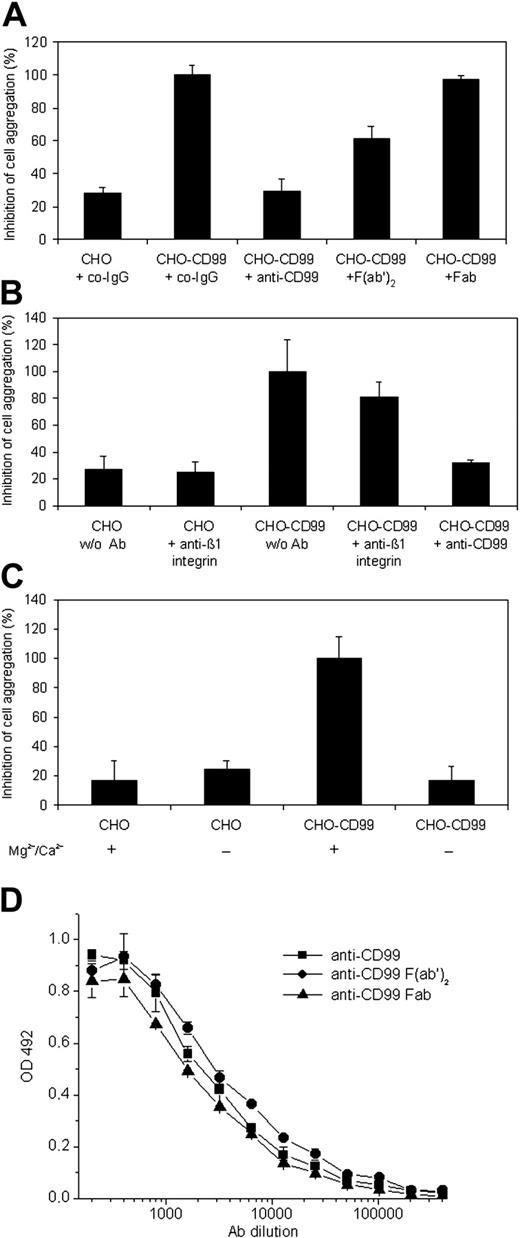
In order to test whether mouse CD99 can support cell adhesion, we generated CHO cells stably expressing this antigen. Although CD99 is only a short transmembrane protein and highly glycosylated, CD99-transfected CHO cells aggregated rather efficiently and CD99-dependent aggregation was blocked with affinity-purified anti-CD99 antibodies, but not with control IgG from the preimmune serum or a mAb against β1-integrin (Figure 3A-B). Surprisingly, F(ab′)2 fragments of anti-CD99 antibodies inhibited only partially and Fab fragments did not block aggregation (Figure 3A), although they bound to CD99-Fc in ELISA assays as well as the complete intact IgG molecules (Figure 3D). CD99-dependent aggregation was blocked in the absence of divalent cations (Figure 3C).
CD99 supports aggregation of CD99-transfected CHO cells. Mock-transfected CHO cells (CHO) or CD99-transfected CHO cells (CHO-CD99) were allowed to aggregate (A) in the presence of 30 μg/mL of the following antibodies: control preimmune IgG (co-IgG), affinity-purified antibodies against mouse CD99 (anti-CD99), F(ab′)2, or Fab fragments of affinity-purified anti-CD99 antibodies (as indicated); P < .0001, CHO-CD99 + co-IgG versus CHO + co-IgG; P < .0001, CHO-CD99 + anti-CD99 versus CHO-CD99 + co-IgG; P < .0005, CHO-CD99 + F(ab′)2 versus CHO-CD99 + co-IgG; P = .25, CHO-CD99 + Fab versus CHO-CD99 + co-IgG. (B) Mock-transfected CHO cells (CHO) or CD99-transfected CHO cells (CHO-CD99) were allowed to aggregate in the absence of antibodies (w/o Ab), or in the presence of affinity-purified anti-CD99 antibodies or control antibodies against β1-integrin (as indicated); P < .01, CHO-CD99 + anti-CD99 versus CHO-CD99 w/o Ab; P = .48, CHO-CD99 w/o Ab versus CHO-CD99 + anti–β1-integrin. (C) After cells were harvested in PBS with 5 mM EDTA, they were washed in Mg2+/Ca2+-free HBSS and then allowed to aggregate in HBSS either without (–) or with (+) 1 mM Mg2+/Ca2+; P < .001, CHO-CD99 without Mg2+/Ca2+ versus CHO-CD99 with Mg2+/Ca2+. (D) Reactivity of affinity-purified anti-CD99 antibodies (anti-CD99) and the corresponding F(ab′)2 and Fab fragments with immobilized CD99-Fc in an ELISA assay, which was developed using peroxidase-labeled polyclonal antibodies to rabbit IgG F(ab′)2. Optical density at OD 492 is plotted against antibody concentration. The results are presented as the mean ± SEM, and are representative of at least 3 separate experiments.
CD99 supports aggregation of CD99-transfected CHO cells. Mock-transfected CHO cells (CHO) or CD99-transfected CHO cells (CHO-CD99) were allowed to aggregate (A) in the presence of 30 μg/mL of the following antibodies: control preimmune IgG (co-IgG), affinity-purified antibodies against mouse CD99 (anti-CD99), F(ab′)2, or Fab fragments of affinity-purified anti-CD99 antibodies (as indicated); P < .0001, CHO-CD99 + co-IgG versus CHO + co-IgG; P < .0001, CHO-CD99 + anti-CD99 versus CHO-CD99 + co-IgG; P < .0005, CHO-CD99 + F(ab′)2 versus CHO-CD99 + co-IgG; P = .25, CHO-CD99 + Fab versus CHO-CD99 + co-IgG. (B) Mock-transfected CHO cells (CHO) or CD99-transfected CHO cells (CHO-CD99) were allowed to aggregate in the absence of antibodies (w/o Ab), or in the presence of affinity-purified anti-CD99 antibodies or control antibodies against β1-integrin (as indicated); P < .01, CHO-CD99 + anti-CD99 versus CHO-CD99 w/o Ab; P = .48, CHO-CD99 w/o Ab versus CHO-CD99 + anti–β1-integrin. (C) After cells were harvested in PBS with 5 mM EDTA, they were washed in Mg2+/Ca2+-free HBSS and then allowed to aggregate in HBSS either without (–) or with (+) 1 mM Mg2+/Ca2+; P < .001, CHO-CD99 without Mg2+/Ca2+ versus CHO-CD99 with Mg2+/Ca2+. (D) Reactivity of affinity-purified anti-CD99 antibodies (anti-CD99) and the corresponding F(ab′)2 and Fab fragments with immobilized CD99-Fc in an ELISA assay, which was developed using peroxidase-labeled polyclonal antibodies to rabbit IgG F(ab′)2. Optical density at OD 492 is plotted against antibody concentration. The results are presented as the mean ± SEM, and are representative of at least 3 separate experiments.
Although homotypic cell aggregation was CD99 dependent, direct binding of CD99 molecules to each other could not be demonstrated. Binding of CD99-Fc to CD99-expressing CHO cells or lymphocytes was not detected by fluorescence-activated cell-sorter (FACS) analysis (not shown). Likewise, CD99-transfected CHO cells did not adhere to immobilized CD99-Fc (data not shown). This does not necessarily rule out that CD99 molecules could bind to each other, since VE-cadherin-Fc and VE-cadherin–transfected CHO behaved similarly in FACS analysis (not shown) and in adhesion assays (data not shown), whereas P-selectin glycoprotein ligand 1 (PSGL-1)–expressing CHO cells (coexpressing the appropriate glycosyltransferases) bound very efficiently to P-selectin–Fc. However, in combination with the lack of Fab fragments to inhibit cell aggregation, homophilic binding of CD99 in these assays is not likely. If CD99 interacts in a homophilic way, it may require appropriate spatial arrangements of the molecules in cell membranes. Indeed, culturing a mixture of CHO cells and CD99-transfected CHO cells allowed the detection of CD99 preferentially between cells that both expressed CD99, whereas CD99 was often, although not always, missing at cell contacts if the neighboring cell did not express CD99 (see Supplemental Figure 1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
Antibodies against mouse CD99 inhibit TEM of lymphocytes in vitro
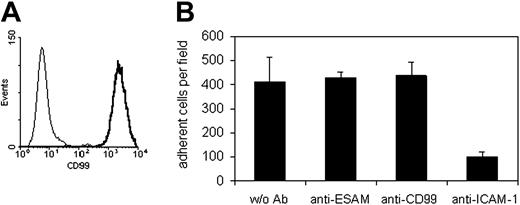
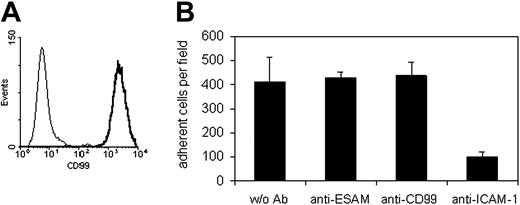
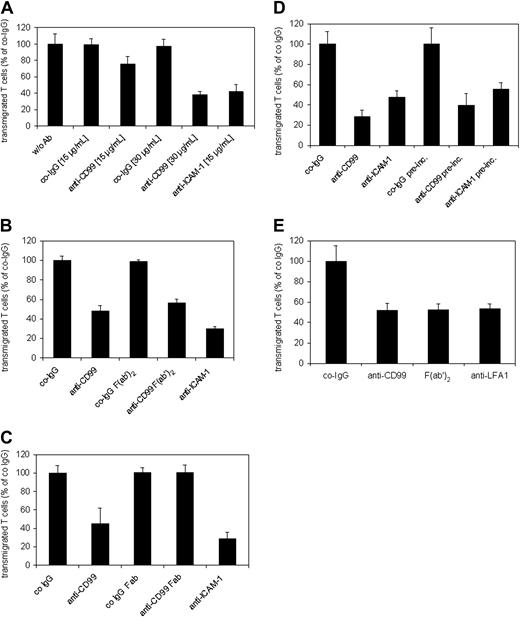
In order to analyze whether CD99 is involved in interactions of T cells with endothelial cells, we analyzed the antigen-specific T-cell line SJL.PLP7. This cell line expressed CD99 at the cell surface (Figure 4A) and has been demonstrated to migrate efficiently through cell layers of the mouse bEnd.5 cells.47 In all adhesion and TEM experiments, this and another cell line (SJL.PLP3) behaved similarly, therefore, results will be shown only for SJL.PLP7. Analyzing adhesion of these T cells to bEnd.5 cells grown on fibronectin-coated glass chamber slides and stimulated for 16 hours with TNF-α prior to the assay revealed that our blocking antibodies against CD99 did not interfere with adhesion of the lymphocytes to the endothelial cell monolayer, whereas adhesion was efficiently blocked with a mAb against ICAM-1 (Figure 4B). Lack of inhibition of cell adhesion had been expected, since CD99 was absent mainly from the apical surface and highly enriched at endothelial cell contacts (Figure 2B). However, at the same antibody concentration (30 μg/mL) anti-CD99 antibodies blocked the transmigration of SJL.PLP7 cells through a monolayer of bEnd.5 cells grown on transwell filters by 61.5% (± 3.6%) (Figure 5A). No inhibition was seen with control IgG from the preimmune serum. In order to exclude that potential interactions of the intact anti-CD99 antibodies with Fc receptors on the T cells would interfere with our results, we analyzed the effect of F(ab′)2 fragments. As shown in Figure 5B, 30 μg/mL F(ab′)2 fragments inhibited transmigration similarly as the same concentration of intact anti-CD99 IgG. However, Fab fragments of anti-CD99 antibodies did not block transmigration (Figure 5C), arguing for a role of CD99 in TEM independent of homophilic interactions.
The CD99-expressing antigen-specific memory/effector TH1cell line SJL.PLP7 does not bind via CD99 to mouse bEnd.5 endothelioma cells. (A) Binding of anti-CD99 antibodies (solid line) and control IgG (thin line) to SJL.PLP7 cells as analyzed by flow cytometry. (B) SJL.PLP7 cells were allowed to adhere to bEnd.5 cells in the absence of antibodies (w/o Ab), or in the presence of 30 μg/mL antibodies against ESAM (anti-ESAM), against CD99 (anti-CD99), or against ICAM-1 (anti–ICAM-1). P < .005, anti–ICAM-1 versus w/o Ab. The results are presented as the mean ± SEM, and are representative of at least 3 separate experiments.
The CD99-expressing antigen-specific memory/effector TH1cell line SJL.PLP7 does not bind via CD99 to mouse bEnd.5 endothelioma cells. (A) Binding of anti-CD99 antibodies (solid line) and control IgG (thin line) to SJL.PLP7 cells as analyzed by flow cytometry. (B) SJL.PLP7 cells were allowed to adhere to bEnd.5 cells in the absence of antibodies (w/o Ab), or in the presence of 30 μg/mL antibodies against ESAM (anti-ESAM), against CD99 (anti-CD99), or against ICAM-1 (anti–ICAM-1). P < .005, anti–ICAM-1 versus w/o Ab. The results are presented as the mean ± SEM, and are representative of at least 3 separate experiments.
Blocking of CD99 inhibits TEM of SJL.PLP7 cells. T cells were allowed to transmigrate through the monolayer of bEnd.5 cells grown on transwell filters, either for 2 hours in the absence of the chemokine SDF-1 (A-C), or for 30 minutes in the presence of 100 ng/mL SDF-1 in the lower chamber (C-D). (A) Endothelial cells were incubated for 30 minutes prior to the start of the assay either without antibodies (w/o Ab) or with the indicated concentrations of preimmune control IgG (co-IgG), affinity-purified anti-CD99 IgG (anti-CD99), or a mAb against ICAM-1 (anti–ICAM-1). Antibodies remained present during the assay. P < .005, anti-CD99 (15 μg/mL) versus co-IgG (15 μg/mL); P < .0005, anti-CD99 (30 μg/mL) versus co-IgG (30 μg/mL) or anti–ICAM-1 (15 μg/mL) versus co-IgG (15 μg/mL). (B) Endothelial cells were pretreated with 30 μg/mL preimmune IgG (co-IgG), intact anti-CD99 antibodies (anti-CD99), F(ab′)2 fragments of control IgG or of anti-CD99 IgG, or with a mAb against ICAM-1 (anti–ICAM-1). As in panel A, antibodies remained present during the assay. P < .0005, co-IgG versus anti-CD99 or anti–ICAM-1; P < .0005, anti-CD99 F(ab′)2 versus co-IgG F(ab′)2. (C) Endothelial cells were pretreated with 30 μg/mL preimmune IgG (co-IgG), intact anti-CD99 antibodies (anti-CD99), Fab fragments of control IgG (co-IgG Fab) or of anti-CD99 IgG (anti-CD99 Fab), or with a mAb against ICAM-1 (anti–ICAM-1). As in panels A-C, antibodies remained present during the assay. P < .01, anti-CD99 versus co-IgG; P < .001, anti–ICAM-1 versus co-IgG. (D) After preincubation of the endothelial cells with 30 μg/mL of the indicated antibodies, the antibodies either remained present during the assay (first 3 bars) or were washed away before the addition of the lymphocytes (indicated by “pre-inc.” underneath the last 3 bars). P < .001, anti-CD99 versus co-IgG; P < .005, anti–ICAM-1 versus co-IgG; P < .005, co-IgG pre-inc. versus anti-CD99 preinc. or anti–ICAM-1 pre-inc. (E) Lymphocytes were preincubated with 30 μg/mL preimmune control IgG, anti-CD99 IgG or a mAb against LFA-1, or with F(ab′)2 fragments of anti-CD99 antibodies. Antibodies and F(ab′)2 fragments were washed away prior to adding the lymphocytes into the assay. Each panel is representative of at least 5 experiments; each measurement was done in triplicate. P < .001, co-IgG versus anti-CD99 or F(ab′)2 or anti–LFA-1. Results are presented as mean ± SEM, and are representative of at least 3 experiments.
Blocking of CD99 inhibits TEM of SJL.PLP7 cells. T cells were allowed to transmigrate through the monolayer of bEnd.5 cells grown on transwell filters, either for 2 hours in the absence of the chemokine SDF-1 (A-C), or for 30 minutes in the presence of 100 ng/mL SDF-1 in the lower chamber (C-D). (A) Endothelial cells were incubated for 30 minutes prior to the start of the assay either without antibodies (w/o Ab) or with the indicated concentrations of preimmune control IgG (co-IgG), affinity-purified anti-CD99 IgG (anti-CD99), or a mAb against ICAM-1 (anti–ICAM-1). Antibodies remained present during the assay. P < .005, anti-CD99 (15 μg/mL) versus co-IgG (15 μg/mL); P < .0005, anti-CD99 (30 μg/mL) versus co-IgG (30 μg/mL) or anti–ICAM-1 (15 μg/mL) versus co-IgG (15 μg/mL). (B) Endothelial cells were pretreated with 30 μg/mL preimmune IgG (co-IgG), intact anti-CD99 antibodies (anti-CD99), F(ab′)2 fragments of control IgG or of anti-CD99 IgG, or with a mAb against ICAM-1 (anti–ICAM-1). As in panel A, antibodies remained present during the assay. P < .0005, co-IgG versus anti-CD99 or anti–ICAM-1; P < .0005, anti-CD99 F(ab′)2 versus co-IgG F(ab′)2. (C) Endothelial cells were pretreated with 30 μg/mL preimmune IgG (co-IgG), intact anti-CD99 antibodies (anti-CD99), Fab fragments of control IgG (co-IgG Fab) or of anti-CD99 IgG (anti-CD99 Fab), or with a mAb against ICAM-1 (anti–ICAM-1). As in panels A-C, antibodies remained present during the assay. P < .01, anti-CD99 versus co-IgG; P < .001, anti–ICAM-1 versus co-IgG. (D) After preincubation of the endothelial cells with 30 μg/mL of the indicated antibodies, the antibodies either remained present during the assay (first 3 bars) or were washed away before the addition of the lymphocytes (indicated by “pre-inc.” underneath the last 3 bars). P < .001, anti-CD99 versus co-IgG; P < .005, anti–ICAM-1 versus co-IgG; P < .005, co-IgG pre-inc. versus anti-CD99 preinc. or anti–ICAM-1 pre-inc. (E) Lymphocytes were preincubated with 30 μg/mL preimmune control IgG, anti-CD99 IgG or a mAb against LFA-1, or with F(ab′)2 fragments of anti-CD99 antibodies. Antibodies and F(ab′)2 fragments were washed away prior to adding the lymphocytes into the assay. Each panel is representative of at least 5 experiments; each measurement was done in triplicate. P < .001, co-IgG versus anti-CD99 or F(ab′)2 or anti–LFA-1. Results are presented as mean ± SEM, and are representative of at least 3 experiments.
Assays described thus far were performed in the presence of anti-CD99 antibodies. In order to test whether CD99 on the T cells or on the endothelial cells participates in the TEM process, either the endothelial cell monolayer or the T cells were preincubated with 30 μg/mL of the inhibitory antibodies for 30 minutes at 37°C and then washed, and transmigration was subsequently investigated in the absence of antibodies. If the transmigration assays were performed for 2 hours, the inhibitory effect of the antibodies was either completely lost (preincubation of T cells) or reduced by about 50% (preincubation of endothelial cells) when compared with assays with antibodies still present during transmigration (not shown). This observation is in agreement with the reversibility of the inhibitory effect observed for anti–human CD99 antibodies in TEM assays with human monocytes.18 When the efficiency of transmigration was enhanced by adding the chemokine SDF-1 to the lower chamber of the transwells and assays were performed for only 30 minutes, the anti-CD99 antibodies inhibited transmigration by 71% (± 6%) if the endothelial cell monolayer was preincubated with antibodies and the antibodies remained present during the assay (Figure 5D). If the antibodies were washed away prior to the assay, transmigration was inhibited with similar efficiency (Figure 5D). If only the T lymphocytes were preincubated with antibodies and the antibodies were removed prior to the assay, anti-CD99 antibodies inhibited transmigration by 50% (± 6%), comparable with the inhibitory effect of anti–lymphocyte function–associated antigen 1 (LFA-1) antibodies (46% ± 4.3%) (Figure 5E). We conclude that CD99 on T cells as well as on endothelial cells is involved in TEM of T cells.
Since antibodies against human CD99 have been reported to stimulate homotypic aggregation of lymphocytes, we analyzed SJL.PLP7 cells microscopically for signs of cell aggregation upon preincubation with anti-CD99 antibodies (under conditions similar to those used for transmigration assays). No evidence for cell aggregation was found (not shown).
When TEM assays were performed with mouse peripheral blood mononuclear cells, we found that anti-CD99 antibodies inhibited transmigration of T cells as well as of B cells (data not shown). Similarly, TEM of T cells isolated from lymph nodes of DNFB-treated mice was inhibited by anti-CD99 antibodies (data not shown).
Mouse CD99 participates in the recruitment of lymphocytes into inflamed skin in vivo and in edema formation
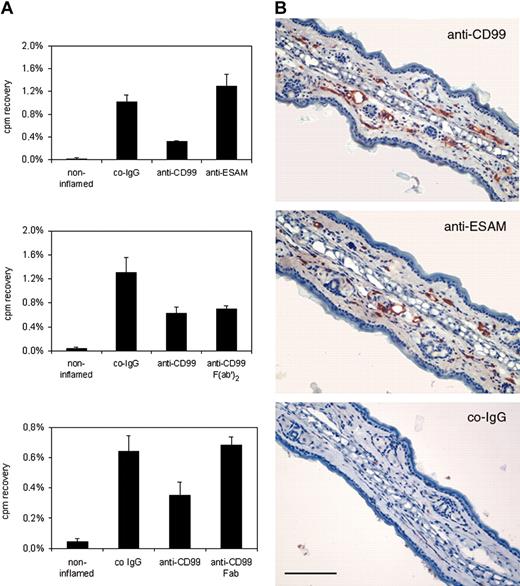
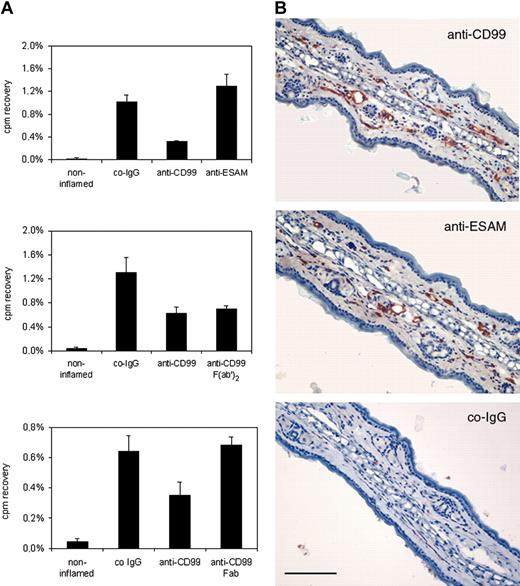
To determine whether CD99 is involved in T-lymphocyte migration into inflamed sites of the skin, we isolated in vivo–activated T cells from lymph nodes of DNFB-treated mice. Isolated cells were radioactively labeled with 51Cr and intravenously injected into mice with a DNFB-elicited DTH reaction in the skin of the right ear. Antibodies against CD99 or control antibodies against the endothelial antigen ESAM were co-injected with T cells. Mice were killed 15 hours after injection, and the distribution of the radioactivity in the inflamed right and noninflamed left ear, as well as in various other organs (spleen, liver, lung, not shown), was measured. As shown in Figure 6A anti-CD99 antibodies inhibited T-cell recruitment into the inflamed ear by 68% (± 1%), whereas no inhibitory effect was seen with preimmune IgG from the same serum or with affinity-purified antibodies against ESAM. Importantly, in a separate experiment, F(ab′)2 fragments from anti-CD99 antibodies had a similar inhibitory effect (47% ± 4.5%) on T-cell immigration into the inflamed ear as did intact IgG (53% ± 8.3%) (Figure 6A), excluding the possibility that interactions with Fc receptors or complement would interfere with our results. Similar to the results in TEM assays, Fab fragments of anti-CD99 antibodies did not block T-cell recruitment (Figure 6A).
Delayed-type hypersensitivity response in the skin is inhibited by anti-CD99 antibodies. (A) Blocking of CD99 partially inhibits immigration of T cells into inflamed skin. Radiolabeled in vivo–activated T cells were injected together with 70 μg control IgG from preimmune serum (co-IgG), affinity-purified antibodies against CD99 (anti-CD99), and affinity-purified antibodies against ESAM (anti-ESAM), 70 μg F(ab′)2 fragments (anti-CD99 F(ab′)2, or 70 μg Fab fragments (anti-CD99 Fab) of affinity-purified antibodies against CD99. Immigration of cells into the noninflamed control ears is depicted by the first bar (noninflamed). Results in the upper 2 panels are representative of 5 similar experiments; those in the bottom panel, of 3 similar experiments. For each determination, 4 mice were analyzed. Experiments shown by the 2 graphs were performed with 2 different preparations of T cells. Numbers on the left refer to the percentage of injected cells that were found in the analyzed ear. P < .001, anti-CD99 versus co-IgG (upper panel); P < .005, co-IgG versus anti-CD99 or F(ab′)2 (middle panel); P < .001, anti-CD99 versus co-IgG (bottom panel). Results are presented as mean ± SEM and are representative of at least 3 separate experiments. (B) CD99 and ESAM are accessible for antibodies from within the blood vessel lumen. DNFB-sensitized and -challenged mice were injected either with affinity-purified antibodies against CD99 or against ESAM, or with control IgG from rabbit preimmune serum (as indicated). Then, 15 minutes later anesthetized mice were perfused with PBS to remove unbound antibody and then with PFA to fix bound antibody. Cryostat sections of the inflamed ear were incubated with secondary antibodies and stained as described in “Materials and methods.” Bar represents 200 μm.
Delayed-type hypersensitivity response in the skin is inhibited by anti-CD99 antibodies. (A) Blocking of CD99 partially inhibits immigration of T cells into inflamed skin. Radiolabeled in vivo–activated T cells were injected together with 70 μg control IgG from preimmune serum (co-IgG), affinity-purified antibodies against CD99 (anti-CD99), and affinity-purified antibodies against ESAM (anti-ESAM), 70 μg F(ab′)2 fragments (anti-CD99 F(ab′)2, or 70 μg Fab fragments (anti-CD99 Fab) of affinity-purified antibodies against CD99. Immigration of cells into the noninflamed control ears is depicted by the first bar (noninflamed). Results in the upper 2 panels are representative of 5 similar experiments; those in the bottom panel, of 3 similar experiments. For each determination, 4 mice were analyzed. Experiments shown by the 2 graphs were performed with 2 different preparations of T cells. Numbers on the left refer to the percentage of injected cells that were found in the analyzed ear. P < .001, anti-CD99 versus co-IgG (upper panel); P < .005, co-IgG versus anti-CD99 or F(ab′)2 (middle panel); P < .001, anti-CD99 versus co-IgG (bottom panel). Results are presented as mean ± SEM and are representative of at least 3 separate experiments. (B) CD99 and ESAM are accessible for antibodies from within the blood vessel lumen. DNFB-sensitized and -challenged mice were injected either with affinity-purified antibodies against CD99 or against ESAM, or with control IgG from rabbit preimmune serum (as indicated). Then, 15 minutes later anesthetized mice were perfused with PBS to remove unbound antibody and then with PFA to fix bound antibody. Cryostat sections of the inflamed ear were incubated with secondary antibodies and stained as described in “Materials and methods.” Bar represents 200 μm.
Accessibility of endothelial CD99 and of the endothelial control antigen ESAM on the endothelial cell surface for antibodies from within the blood vessel lumen was analyzed by injecting the antibodies into living mice. Animals were anesthetized 15 minutes later, perfused with PBS to remove unbound antibodies, and then perfused with paraformaldehyde to fix bound antibodies. Tissue sections were processed for immunohistochemistry omitting the first antibody. In this way we could show that both antigens were stained with antibodies from within blood vessels of inflamed ears and no staining was obtained with control IgG from preimmune serum (Figure 6B).
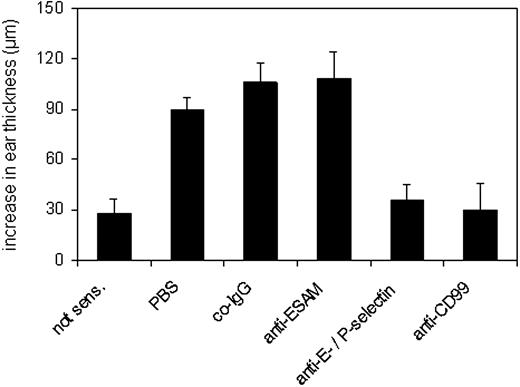
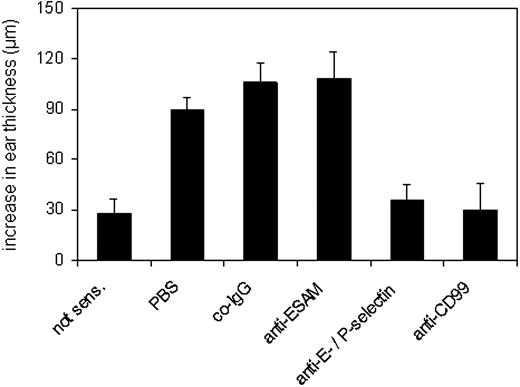
The inhibitory effect of the anti-CD99 antibodies on T-cell accumulation in the skin encouraged us to test whether the antibodies would even inhibit edema formation. To this end, antibodies were injected immediately prior to challenging the ear skin with DNFB. Ear swelling was measured 10 hours after challenge. As shown in Figure 7, affinity-purified anti-CD99 antibodies inhibited antigen-dependent ear swelling almost completely and with similar efficiency as the combination of anti–E-selectin and anti–P-selectin antibodies (Figure 7). Residual swelling was similar as determined for DNFB-treated ears of mice not sensitized prior to challenge (Figure 7). No reduction of ear swelling was seen with preimmune rabbit IgG or with affinity-purified antibodies against ESAM (Figure 7). Peripheral leukocyte counts were unaffected by the anti-CD99 antibodies and were determined as 4.91 ± 0.8 × 103/μL for the PBS control, 4.32 ± 0.89 × 103/μL for rabbit IgG, and 4.43 ± 0.56 × 103/μL for affinity-purified anti-CD99 IgG.
Blocking CD99 inhibits ear swelling. Mice were either not sensitized (first bar, not sens.) or sensitized with DNFB (all other bars) 5 days prior to challenging one of the ears with DNFB. Immediately prior to challenging, mice were intravenously injected with 100 μg anti-CD99 or 150 μg anti–E- and anti–P-selectin antibodies in PBS or with PBS alone (as indicated). Rabbit preimmune IgG was used as negative control IgG (co-IgG). The ear swelling response 10 hours after challenge is expressed as the difference (μm) between the thickness of the challenged ear and the thickness of the vehicle-treated ear. Results are representative for 3 similar experiments; for each determination 5 mice were analyzed. P < .000 05, co-IgG versus anti-CD99 or anti–E-/P-selectin. Results are presented as mean ± SEM and are representative of at least 3 separate experiments.
Blocking CD99 inhibits ear swelling. Mice were either not sensitized (first bar, not sens.) or sensitized with DNFB (all other bars) 5 days prior to challenging one of the ears with DNFB. Immediately prior to challenging, mice were intravenously injected with 100 μg anti-CD99 or 150 μg anti–E- and anti–P-selectin antibodies in PBS or with PBS alone (as indicated). Rabbit preimmune IgG was used as negative control IgG (co-IgG). The ear swelling response 10 hours after challenge is expressed as the difference (μm) between the thickness of the challenged ear and the thickness of the vehicle-treated ear. Results are representative for 3 similar experiments; for each determination 5 mice were analyzed. P < .000 05, co-IgG versus anti-CD99 or anti–E-/P-selectin. Results are presented as mean ± SEM and are representative of at least 3 separate experiments.
Discussion
CD99, a long-known cell-surface antigen on human erythrocytes and most leukocytes, was only recently identified on human endothelial cells and reported to be involved in the in vitro transmigration of monocytes through human endothelial cell monolayers.18 We report here the cloning and first functional analysis of a mouse membrane protein that shares essential structural and functional characteristics with human CD99. First, its amino acid sequence is 45% identical to the shorter of the 2 splice variants of human CD99. Second, it is expressed on mouse peripheral blood T and B cells, neutrophils, monocytes, and dendritic cells as well as at interendothelial cell contacts. Third, despite its small size and O-glycosylation it is able to support homotypic aggregation of transfected cells, a function that is completely blocked by anti-CD99 antibodies. Fourth, these antibodies interfere with leukocyte diapedesis. Based on this evidence, we conclude that we have identified the mouse counterpart of human CD99. Importantly, our results provide the first evidence for the relevance of CD99 during TEM of TH1 memory/effector cells in vitro as well as for the in vivo recruitment of in vivo–activated T cells into inflamed tissue and for the elicitation of a DTH reaction in the skin. This establishes CD99 for the first time as a valid in vivo target for the interference with inflammatory reactions at least in the skin.
Except for the function of human CD99 in the diapedesis of monocytes through cultured monolayers of human endothelial cells, the function of CD99 has been studied mainly on thymocytes and T cells. Various functional effects were attributed to CD99, based on ligation of the antigen with antibodies. For CD4+CD8+ thymocytes and Jurkat cells, but not for peripheral T cells, homotypic aggregation was reported to be triggered by anti-CD99 antibodies, and aggregation was found to be independent of β1- or β2-integrins.21 On the other hand, ligation of CD99 on Jurkat cells and on activated, but not on naive, peripheral T cells was shown to activate the integrin α4β1 resulting in increased T-cell interactions with VCAM-1 under flow.23 Activation of the integrin αLβ2 was excluded in this report. In contrast to these results, activation of LFA-1/ICAM-1–mediated homotypic cell aggregation upon super cross-linking of CD99 with first and secondary antibodies for more than 4 hours was reported for the B lymphoblastoid cell line IM-9.22
We consider it unlikely that the activation of ICAM-1– and VCAM-1–binding integrins would be related to the inhibitory effects of anti-CD99 antibodies on the TEM of antigen-specific T lymphocytes for the following reasons: First, we found no evidence for homotypic aggregation of T lymphocytes upon preincubation with our anti-CD99 antibodies. Second, adhesion of T lymphocytes to the TNF-α–stimulated endothelial cell monolayer grown on lab-tek slides was not enhanced upon preincubation of T cells with the anti-CD99 antibodies. Since ICAM-1 and VCAM-1 are strongly induced on bEnd.5 cells upon stimulation with TNF-α,34 we conclude that our anti-CD99 antibodies did not activate the integrins LFA-1 or α4β1 on our T lymphocytes. Third, adhesion of lymphocytes to ICAM-1–transfected CHO cells was neither increased nor inhibited by anti-CD99 antibodies, further excluding that anti-CD99 antibodies affect the function of β2-integrins on these cells (Supplemental Figure 2).48 Fourth, it is important to note that TEM of T lymphocytes was already inhibited upon the exclusive binding of antibodies to CD99 on each of the 2 cell types alone. Thus, antibody ligation of either endothelial CD99 or of T lymphocyte CD99 was sufficient to inhibit transmigration.
Although we believe that activation or inactivation of β2-integrins or integrin α4β1 is not involved in the effects we have observed, the detailed molecular mechanism by which anti-CD99 antibodies block TEM of T lymphocytes is still unknown. The fact that TEM of lymphocytes as well as lymphocyte extravasation was inhibited by intact anti-CD99 antibodies and F(ab′)2 fragments, but not by monomeric Fab fragments, indicates that CD99 may not be directly involved as homophilic adhesion molecule. This is in line with our results that direct binding of CD99-expressing cells to CD99-Fc could not be demonstrated and that aggregation of CD99-expressing CHO cells was blocked only by anti-CD99 IgG but not by anti-CD99 Fab fragments.
However, homophilic CD99 interactions with low affinity cannot completely be ruled out. Indeed, CD99 was concentrated at cell contacts of transfected CHO cells, especially between cells that both expressed CD99, but often not at cell contacts if the neighboring cell lacked CD99 (Supplemental Figure 1). It is possible that CD99 interactions require certain spatial arrangements of CD99 within the plasma membrane to enhance avidity. In contrast to our results for mouse CD99, Fab fragments of a mAb against human CD99 were able to block monocyte migration through human endothelial cell layers, suggesting a direct involvement of CD99 with homophilic or heterophilic ligands.18 In addition, human CD99 has been demonstrated to support aggregation of transfected L cells with each other, but not with mock-transfected L cells.18
Whatever will be the mechanism by which CD99 participates in diapedesis, our results on mouse CD99 as well as those on human endothelial CD9918 establish that antibody ligation of CD99 does not affect the docking of human monocyte or mouse lymphocytes to endothelial cells, but does affect the subsequent diapedesis step. In addition, CD99 on the leukocyte side as well as on endothelium is involved in this process. Heterophilic ligands for CD99 could be involved. In fact, the paired immunoglobulin-like receptor β (PILRβ) has recently been described as a heterophilic ligand for CD99, although, this ligand has so far been found only on natural killer cells, macrophages, and dendritic cells.46 Besides heterophilic ligands in trans, membrane proteins associating with CD99 in cis on the plasma membrane of endothelial cells as well as on lymphocytes could be targets for CD99-mediated effects in the diapedesis process. Future studies will be necessary to reveal the molecular mechanism by which CD99 participates in lymphocyte diapedesis.
Interestingly, the function of CD99 in diapedesis is specific for the homing of T cells into inflamed tissue, since homing of lymphocytes to lymph nodes was not inhibited by anti-CD99 antibodies (Supplemental Figure 3).49 Thus CD99 acts in an inflammation-specific manner and supports lymphocyte diapedesis only in the right endothelial and lymphocyte context, possibly requiring inflammatory activation.
With the identification of mouse CD99 and the generation of diapedesis-blocking reagents, it will now be possible to further analyze this new molecular player in TEM of lymphocytes in vivo. It is intriguing that the anti-CD99 antibodies could block not only the accumulation of labeled T cells in inflamed skin, but even edema formation, pointing to a central role for CD99 in the inflammatory process. It will be important to analyze its relevance for other leukocyte types such as neutrophils and monocytes and to study its role in different in vivo models of inflammation. In summary, our results establish for the first time that the endothelial cell contact–associated membrane protein CD99 is involved in T-lymphocyte extravasation in vivo. This establishes CD99 as a valid pharmacologic target for the treatment of inflammatory processes in the skin.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-03-1184.
Supported by a grant from the Interdisciplinary Center of Clinical Research Münster (Project 2-006).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Chiara Uboldi for providing and culturing the SJL.PLP-3 cells, to Sebastian Bäumer for the endotoxin determinations, and to Urban Deutsch for help with the DNA sequence analysis.