Abstract
Vascular endothelial activation is an early step during leukocyte/endothelial adhesion and transendothelial leukocyte migration in inflammatory states. Leukocyte transmigration occurs through intercellular gaps between endothelial cells. Vascular endothelial cadherin (VE-cadherin) is a predominant component of endothelial adherens junctions that regulates intercellular gap formation. We found that tumor necrosis factor (TNF) caused tyrosine phosphorylation of VE-cadherin, separation of lateral cell-cell junctions, and intercellular gap formation in human umbilical vein endothelial cell (HUVEC) monolayers. These events appear to be regulated by intracellular oxidant production through endothelial NAD(P)H (nicotinamide adenine dinucleotide phosphate) oxidase because antioxidants and expression of a transdominant inhibitor of the NADPH oxidase, p67(V204A), effectively blocked the effects of TNF on all 3 parameters of junctional integrity. Antioxidants and p67(V204A) also decreased TNF-induced JNK activation. Dominant-negative JNK abrogated VE-cadherin phosphorylation and junctional separation, suggesting a downstream role for JNK. Finally, adenoviral delivery of the kinase dead PAK1(K298A) decreased TNF-induced JNK activation, VE-cadherin phosphorylation, and lateral junctional separation, consistent with the proposed involvement of PAK1 upstream of the NADPH oxidase. Thus, PAK-1 acts in concert with oxidase during TNF-induced oxidant production and loss of endothelial cell junctional integrity.
Introduction
Vascular endothelial activation is a hallmark of inflammatory and prothrombotic states. Cytokine-mediated endothelial signaling is a crucial aspect of such activation and usually results in leukocyte adhesion and transmigration through intercellular gaps within the endothelium. Although this process is well described, the signals that regulate intercellular gap formation and separation of lateral cell-cell junctions between endothelial cells are only partly understood. Cytokines have long been implicated during vascular dysfunction; however, the intracellular mediators of cytokine signaling are still under investigation. Recent studies suggest that intracellular oxidants are putative mediators of cytokine signaling. Further, antioxidants prevent tumor necrosis factor (TNF)–mediated NFκB and p38 activation.1,2 A particularly intriguing discovery is the identification of NADPH (nicotinamide adenine dinucleotide phosphate) oxidase subunits in nonphagocytic cells, including smooth muscle and endothelium.3-7 The oxidant-generating machinery in these cells appears to produce oxidants that function as intracellular signaling molecules. However, the mechanisms underlying intracellular oxidant production are undetermined. In addition, the specific reactive oxygen species (ROS) produced during cytokine exposure and the temporal pattern and duration of endothelial oxidant production are unclear. Analogs of the cytosolic and membranous components of NADPH oxidase have been identified in endothelial cells.6,7 This oxidase produces low levels of oxidants in response to stimulation by a variety of agonists.5,8-10 Our goal in this study was to investigate a potential link between endothelial NADPH oxidase and TNF-induced tyrosine phosphorylation of vascular endothelial cadherin (VE-cadherin), junctional dissociation, and endothelial monolayer dysfunction.
We have previously shown that TNF-mediated junctional dissociation and intercellular gap formation are associated with tyrosine phosphorylation of the junctional protein, VE-cadherin.11 The present study suggests that PAK1 and the NADPH oxidase lie upstream of JNK activation and consequent VE-cadherin phosphorylation during TNF-induced junctional separation.
Materials and methods
Cells and media
Human umbilical vein endothelial cells (HUVECs; American Type Culture Collection, Manassas, VA) were grown in endothelial cell growth medium 2 (EGM-2) culture medium (Clonetics, San Diego, CA) containing 2% fetal calf serum and were used between passages 2 and 7. Cells were serum starved for 24 hours before experiments.
Agonists, inhibitors, and antibodies
Recombinant human TNF (rhTNF; Sigma Chemical, St Louis, MO) was used at a concentration of 100 U/mL. Intracellular oxidants were inhibited with N-acetyl-l-cysteine (NAC; Sigma Chemical), superoxide dismutase (SOD)–mimetics, manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), MnTmPyP, (Calbiochem, San Diego, CA), or diphenylene iodonium (DPI). NAC is a free-radical scavenger, whereas MnTBAP and MnTmPyP are cell-permeable SOD mimetics.12 DPI is an NADPH oxidase inhibitor. Human monoclonal antibodies against VE-cadherin and phosphotyrosine were purchased from Cell Signaling Technology (Beverly, MA). Dominant-negative JNK1 plasmid was a kind gift from Dr Lynn Heasley (University of Colorado, Denver, CO).
Cloning, transfection, and infection experiments
Transfection and infection. To increase transfection efficiency, passages 2 to 5 subconfluent HUVECs (ATCC; American Type Culture Collection, Manassas, VA) were synchronized at the G1-S transition with 3.5 mM thymidine overnight. Six hours after thymidine release, cells were incubated with 5 μg plasmid (VE-cadherin, green fluorescence protein [GFP], or dominant-negative JNK), dissolved in TE buffer (pH 7.8) and 30 μL Superfect transfection reagent (Qiagen, Valencia, CA) and incubated for 5 to 10 minutes at room temperature to allow transfection-complex formation. The transfection complex was then transferred to the cell suspension, incubated for 2 to 3 hours in EGM-2 growth medium, and analyzed the following day.
VE-cadherin GFP. Using a HUVEC cDNA library, the coding region of human VE-cadherin lacking the stop codon was amplified (Pfu Turbo; Stratagene, La Jolla, CA) and cloned into pCR4-TOPO (Invitrogen). The insert was ligated into the EcoR1 and BamH1 sites of pEGFP-N3 (Clontech). The resultant construct was confirmed by sequencing.
Adenoviral construction
Adenoviral vectors containing p47(S303304328D), lacZ, p67(V204A), and PAK1(K298A) were constructed as previously described,13,14 and expression was confirmed in HUVECs by immunoblot (not shown). Ad-VE-cadherin GFP was created by subcloning the VE-cadherin GFP into the shuttle vector pCD316(io) (Microbix; Toronto, ON, Canada). After cotransfection with pBHGlox(del)E1,3Cre in 293IQ cells (Microbix), recombinant viruses were harvested, cloned, and titered. Expression was confirmed with microscopy and immunoblot (not shown).
For experiments requiring adenoviral infection, HUVECs were coinfected with either Ad-lacZ, (control) or Adp67V204A and Ad-VE-cadherin GFP (multiplicity of infection [MOI], 100:1) for 18 to 24 hours before experiments were performed.
Detection of intracellular oxidant production
HUVECs were incubated with 10 μg dichlorofluorescein (DCF) for 20 minutes, washed, and examined by laser fluorescence confocal microscopy on a heated stage. These cells were exposed to vehicle or TNF while fluorescence was visualized by time-lapse photography. Oxidant production was also measured by flow cytometry after loading with DCF. For flow cytometry experiments, HUVEC monolayers were washed with Hanks balanced salt solution (HBSS). DCF (final concentration, 10 μM) was added to the cells, which were then incubated for 20 minutes. Cells were washed and incubated with 1 mL OPTI-MED and 20 ng TNF. After a final wash, the cells were trypsinized (1 mL Trypsin/EDTA [ethylenediaminetetraacetic acid]), scraped, and resuspended.
JNK activity assay
Lysates from confluent HUVECs were examined for JNK activity using a kinase assay kit (Cell Signaling Technology). In brief, HUVEC extracts were incubated with a c-Jun fusion protein to pull down JNK. The subsequent kinase reaction was performed with bound JNK in the presence of cold adenosine triphosphate (ATP) using the c-Jun fusion protein as substrate. Ser-63–phosphorylated c-Jun fusion protein was probed with a phospho c-Jun antibody and detected by horseradish peroxidase (HRP) chemiluminescence.
VE-cadherin phosphorylation
Tyrosine phosphorylation of VE-cadherin was examined by immunoprecipitating VE-cadherin from HUVEC lysates using goat polyclonal anti–VE-cadherin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The resultant immunoprecipitates were subjected to immunoblotting for phosphotyrosine. For immunoprecipitation experiments, cells were washed, lysed in cold lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA [ethyleneglycotetraacetic acid], 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM Na3VO4, and 1 μg/mL leupeptin) and were drawn 3 times through a 23-gauge needle. Cells were scraped and centrifuged for 10 minutes at 12 000g at 4°C, and the supernatant was removed. Five hundred microliters cell lysate and 10 μL anti–VE-cadherin antibody were incubated for 2 hours at 4°C. Protein G Sepharose beads (25 μL) were added to the mixture overnight, and then the beads were recovered by microcentrifugation. The resultant pellet was washed twice, resuspended in sodium dodecyl sulfate (SDS) loading buffer, and used for immunoblotting.
For immunoblots, samples containing equal amounts of protein were solubilized in Laemmli sample buffer and separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) (8% polyacrylamide). All samples were transferred onto nitrocellulose membranes and probed with a 1:1000 concentration of the primary antiphosphotyrosine antibody (Santa Cruz Biotechnology) overnight at 4°C. The secondary HRP-conjugated antimouse antibody (1:1000) was added for 30 minutes and detected by enhanced chemiluminescence. Immunoblots were compared by densitometry.
Confocal microscopy
Confluent HUVECs plated on glass chamber slides were exposed to agonists in the presence or absence of inhibitors. After removal of the culture medium, monolayers were fixed with 4% paraformaldehyde and 0.5% Triton. Immunofluorescence staining for VE-cadherin was performed using a primary anti–VE-cadherin antibody and a secondary antibody. Monolayers were then examined at 100 × or 400 × using a Zeiss 410 Axiovert LSM laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany). VE-cadherin trafficking was examined in live cells after coinfection with Ad-VE-cadherin GFP and either Ad-lacZ or Adp67phox. Confocal microscopy was performed in the presence or absence of TNF. Morphologic changes in the monolayer and VE-cadherin trafficking were recorded by time-lapse photography (Zeiss LSM 410; magnification 100×, numerical aperture 1.3). Image processing was performed with Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Endothelial monolayer dysfunction
Endothelial monolayer dysfunction was measured by transmonolayer electrical resistance or fluorescein isothiocyanate (FITC)–labeled dextran flux across intact monolayers. Cells were grown on Transwell chamber inserts (Corning Costar, Cambridge, MA) and were exposed to vehicle or TNF in the presence or absence of the inhibitors described above. Inserts were placed in EVOM chambers (F1; World Precision Instruments, Sarasota, FL) to measure changes in electrical resistance (TER) from 10 minutes to 4 hours. For transmonolayer dextran flux experiments, FITC-labeled dextran was instilled in the upper chamber of the Transwell cup and was assayed from the lower chamber as previously described.11
Statistical analysis
Statistical analysis was performed by using analysis of variance with significance set at a P value of less than .05.
Results
TNF induces intracellular oxidant production at lateral membrane-associated sites
Exposure of HUVECs to TNF resulted in oxidant generation within 5 minutes. Areas of oxidant production were focal and localized to the cell periphery, suggesting that oxidant production occurred at membrane-associated sites. Oxidant production peaked at 10 minutes after TNF exposure and disappeared by 15 minutes (Figure 1A-D). Oxidant production was also measured by flow cytometry, which showed early TNF-induced oxidant production (Figure 1E). We next examined the role of endothelial NADPH oxidase using DPI, which prevented TNF-induced oxidant production at 5 minutes (P < .05) (Figure 1E).
HUVEC intracellular oxidant production in response to TNF. Live, unfixed HUVECs (original magnification, × 100) loaded with dichlorofluorescein (10 μg/mL DCF) were exposed to vehicle (A), or TNF (100 μ/mL) (B-D) for up to 15 minutes and were observed by confocal microscopy using a heated stage. Oxidant production, as evidenced by fluorescence (arrows), was discrete and localized to membrane sites. This observation was (B) present within 5 minutes of TNF exposure, (C) became maximal at 10 minutes, and (D) disappeared within 15 minutes. (E) Oxidant production at 5 minutes was also measured by flow cytometry, which showed that TNF significantly increased oxidant production (▪) compared with vehicle controls (□). This increase in oxidant production was prevented by DPI (▦).
HUVEC intracellular oxidant production in response to TNF. Live, unfixed HUVECs (original magnification, × 100) loaded with dichlorofluorescein (10 μg/mL DCF) were exposed to vehicle (A), or TNF (100 μ/mL) (B-D) for up to 15 minutes and were observed by confocal microscopy using a heated stage. Oxidant production, as evidenced by fluorescence (arrows), was discrete and localized to membrane sites. This observation was (B) present within 5 minutes of TNF exposure, (C) became maximal at 10 minutes, and (D) disappeared within 15 minutes. (E) Oxidant production at 5 minutes was also measured by flow cytometry, which showed that TNF significantly increased oxidant production (▪) compared with vehicle controls (□). This increase in oxidant production was prevented by DPI (▦).
Antioxidants prevent TNF-induced intercellular gap formation and endothelial barrier dysfunction
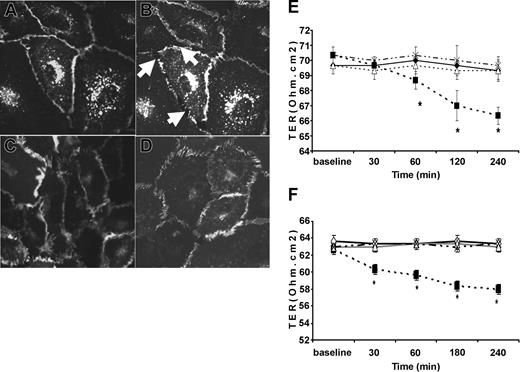
Exposure of HUVECs to TNF resulted in early intercellular gap formation and redistribution of VE-cadherin from intercellular junctions (Figure 2B, arrows). Pre-exposure of HUVEC monolayers to either NAC (Figure 2C) or MnTBAP (Figure 2D) for 30 minutes before TNF exposure effectively prevented TNF-induced intercellular gap formation and redistribution of VE-cadherin from cell-cell junctions (Figure 2C-D). Similarly, TNF significantly decreased TER. Pre-exposure of confluent monolayers to NAC (Figure 2E) or MnTBAP (Figure 2F) prevented TNF-induced decreased TER. Neither NAC nor MnTBAP alone had any effect on baseline TER (Figure 2E-F).
Intercellular gap formation induced by TNF and the effect of antioxidants on TNF-induced TER. Live, unfixed HUVECs (original magnification, × 100) mounted on a heated stage were infected with Ad-VE-cadherin GFP and exposed to vehicle (A) or TNF (B) and were observed under fluorescence confocal microscopy for 30 minutes. Ad-VE-cadherin GFP localized to adherens junctions similar to endogenous VE-cadherin. Compared with vehicle controls (A), TNF caused early separation of cell-cell junctions (B, arrows). In parallel, intercellular gap formation was associated with loss of VE-cadherin GFP from adherens junctions. (C-D) Confluent HUVEC monolayers exposed to the antioxidants (C) NAC (20 mM) or (D) MnTBAP (10 mM) for 30 minutes before TNF exposure. Both NAC and MnTBAP prevented TNF-induced intercellular gap formation and VE-cadherin redistribution. (E-F) Effect of antioxidants on TNF-induced reduction in transmonolayer resistance, TER, and intercellular gap formation. (E) Early and persistent decrease in TER induced by TNF (▪) compared with vehicle controls (□). NAC (▵) prevented TNF-induced decreased TER, whereas NAC alone (×) had no effect on TER. (F) Similar effects were observed with MnTBAP. *P < .05; n > 6 per group.
Intercellular gap formation induced by TNF and the effect of antioxidants on TNF-induced TER. Live, unfixed HUVECs (original magnification, × 100) mounted on a heated stage were infected with Ad-VE-cadherin GFP and exposed to vehicle (A) or TNF (B) and were observed under fluorescence confocal microscopy for 30 minutes. Ad-VE-cadherin GFP localized to adherens junctions similar to endogenous VE-cadherin. Compared with vehicle controls (A), TNF caused early separation of cell-cell junctions (B, arrows). In parallel, intercellular gap formation was associated with loss of VE-cadherin GFP from adherens junctions. (C-D) Confluent HUVEC monolayers exposed to the antioxidants (C) NAC (20 mM) or (D) MnTBAP (10 mM) for 30 minutes before TNF exposure. Both NAC and MnTBAP prevented TNF-induced intercellular gap formation and VE-cadherin redistribution. (E-F) Effect of antioxidants on TNF-induced reduction in transmonolayer resistance, TER, and intercellular gap formation. (E) Early and persistent decrease in TER induced by TNF (▪) compared with vehicle controls (□). NAC (▵) prevented TNF-induced decreased TER, whereas NAC alone (×) had no effect on TER. (F) Similar effects were observed with MnTBAP. *P < .05; n > 6 per group.
NADPH oxidase inhibition prevents TNF-induced junctional protein phosphorylation and intercellular gap formation
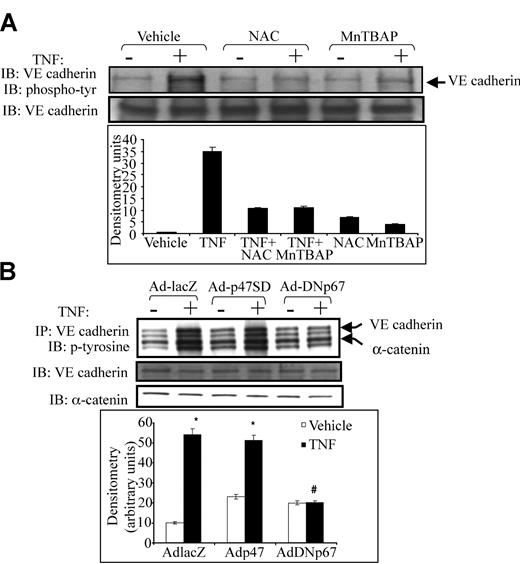
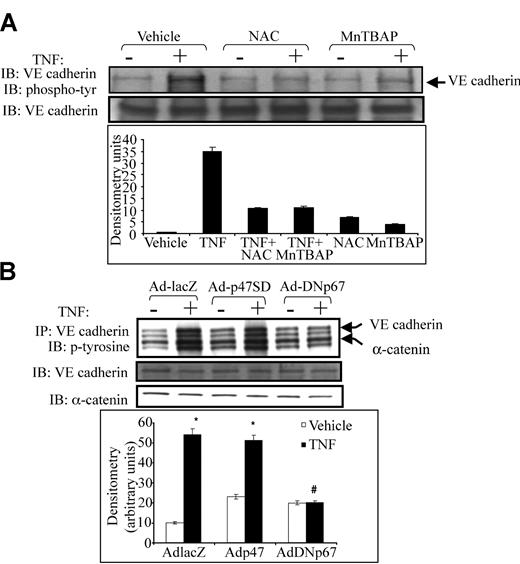
Because tyrosine phosphorylation of VE-cadherin is associated with junctional dissociation, intercellular gap formation, and increased paracellular permeability in vascular endothelium,11,15,16 we examined the effect of antioxidants on VE-cadherin tyrosine phosphorylation. TNF caused robust tyrosine phosphorylation of VE-cadherin. Either NAC or MnTBAP prevented TNF-induced tyrosine phosphorylation of VE-cadherin (Figure 3A). Adding NAC or MnTBAP alone had no effect on VE-cadherin phosphorylation. We also examined the effect of NADPH oxidase inhibition on VE-cadherin phosphorylation by infecting HUVECs with adenovirus containing p67 (Adp67V204A), which harbors an inactivating mutation in the putative activation domain of p67phox.17 Adp67V204A had no effect on basal VE-cadherin phosphorylation but prevented TNF-induced tyrosine phosphorylation of VE-cadherin (Figure 3B), supporting a specific role for the NADPH oxidase in VE-cadherin phosphorylation. Adp67phox (V204A) also prevented TNF-induced α-catenin phosphorylation (Figure 3B). In contrast, the active p47SD mutant, which contains a triple phosphomimetic mutation (S303304, 328D), increased basal VE-cadherin and α-catenin phosphorylation while not affecting TNF-induced junctional protein phosphorylation. The Ad-lacZ control virus had no effect on basal or TNF-stimulated VE-cadherin phosphorylation
Effect of antioxidants and dominant-negative p67phox on tyrosine phosphorylation of VE-cadherin and α-catenin. (A) HUVEC monolayers were exposed to TNF in the presence or absence of NAC or MnTBAP. Lysates were subjected to immunoprecipitation using anti–VE-cadherin antibody. Half the immunoprecipitates were probed with antiphosphotyrosine antibody (top blot), and the other half were probed with anti–VE-cadherin antibody to confirm adequate immunoprecipitation (bottom blot). (A) TNF caused robust tyrosine phosphorylation of VE-cadherin, whereas both NAC and MnTBAP prevented such phosphorylation. (B) HUVECs were infected with Ad-lacZ (control), the active p47phox mutant, Ad-p47SD, or Ad-DNp67 before TNF exposure. Ad-DNp67 prevented TNF-induced tyrosine phosphorylation of VE-cadherin and α-catenin (top panel). Adp47SD caused mild increases in basal VE-cadherin and α-catenin phosphorylation. Histogram (bottom) represents densitometry values for VE-cadherin phosphorylation.
Effect of antioxidants and dominant-negative p67phox on tyrosine phosphorylation of VE-cadherin and α-catenin. (A) HUVEC monolayers were exposed to TNF in the presence or absence of NAC or MnTBAP. Lysates were subjected to immunoprecipitation using anti–VE-cadherin antibody. Half the immunoprecipitates were probed with antiphosphotyrosine antibody (top blot), and the other half were probed with anti–VE-cadherin antibody to confirm adequate immunoprecipitation (bottom blot). (A) TNF caused robust tyrosine phosphorylation of VE-cadherin, whereas both NAC and MnTBAP prevented such phosphorylation. (B) HUVECs were infected with Ad-lacZ (control), the active p47phox mutant, Ad-p47SD, or Ad-DNp67 before TNF exposure. Ad-DNp67 prevented TNF-induced tyrosine phosphorylation of VE-cadherin and α-catenin (top panel). Adp47SD caused mild increases in basal VE-cadherin and α-catenin phosphorylation. Histogram (bottom) represents densitometry values for VE-cadherin phosphorylation.
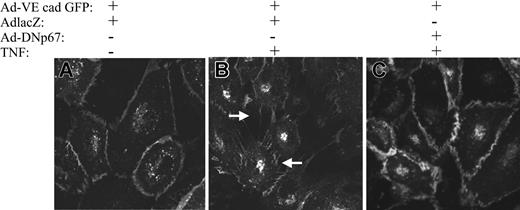
The effect of endothelial NADPH oxidase on TNF-induced redistribution of VE-cadherin and intercellular gap formation was examined in real time by adenoviral coinfection of HUVECs with Ad-VE-cadherin GFP and either Ad-lacZ (control) or Adp67(V204A) (Figure 4A-C). VE-cadherin GFP was localized to cell-cell junctions (Figure 4). Compared with vehicle (4A), TNF caused numerous intercellular gaps in cells infected with Ad-lacZ and Ad-VE-cadherin GFP (4B). Ad-VE-cadherin GFP was redistributed from the cell-cell junctions within 10 minutes of TNF exposure, as were noninfected cells. In contrast, coinfection with Ad-p67V204A prevented intercellular gap formation and redistribution of Ad-VE-cadherin GFP from cell-cell junctions after TNF exposure (Figure 4C).
Effect of AdDNp67 on TNF-induced intercellular gap formation VE-cadherin distribution. Live, unfixed HUVECs (original magnification, × 100) were coinfected with Ad-VE-cadherin GFP (A-C) and either Ad-lacZ (A-B) or Ad-DNp67 (C). Monolayers exposed to vehicle (A) maintained cell-cell contacts, and Ad-VE-cadherin GFP was localized to adherens junctions. In contrast, TNF caused marked cell-cell separation, gap formation, and cadherin redistribution in cells co-infected with Ad-lacZ (B). Ad-DNp67 prevented TNF-induced changes (C).
Effect of AdDNp67 on TNF-induced intercellular gap formation VE-cadherin distribution. Live, unfixed HUVECs (original magnification, × 100) were coinfected with Ad-VE-cadherin GFP (A-C) and either Ad-lacZ (A-B) or Ad-DNp67 (C). Monolayers exposed to vehicle (A) maintained cell-cell contacts, and Ad-VE-cadherin GFP was localized to adherens junctions. In contrast, TNF caused marked cell-cell separation, gap formation, and cadherin redistribution in cells co-infected with Ad-lacZ (B). Ad-DNp67 prevented TNF-induced changes (C).
TNF-induced JNK activation is oxidant-dependent
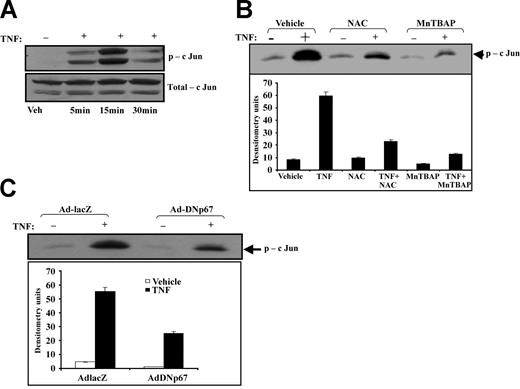
Given that we previously demonstrated that mitogen-activated protein kinase (MAPK) regulates TNF-induced intercellular gap formation and endothelial cell-cell adhesion18 and implicated NADPH oxidase as a mediator of TNF-induced JNK activation,19 we examined the role of oxidants during TNF-induced JNK activation after pretreatment with NAC or MnTBAP. HUVECs demonstrated a 10-fold increase in JNK activity within 15 minutes of TNF exposure (Figure 5A). This observation was transient and disappeared after 30 minutes of TNF exposure. NAC or MnTBAP attenuated TNF-induced JNK activation by 60% and 90%, respectively (Figure 5B). JNK activity in cells exposed to NAC or MnTBAP alone was not significantly different from that of vehicle controls. Infection of HUVECs with dominant-negative Adp67V204A reduced TNF-induced JNK activity by 55% (Figure 5C).
Effect of antioxidants and dominant-negative p67phox on TNF-induced JNK activation. (A) Time-course of JNK activation was first determined by exposing HUVECs to TNF for up to 30 minutes and probing cell lysates for phospho-JNK (p-cJun) and total JNK (total-cJun). JNK activation was apparent within 5 minutes and was maximal by 15 minutes after TNF exposure. (B) HUVEC monolayers exposed to TNF in the presence of NAC or MnTBAP were lysed and examined for JNK activity using a functional JNK assay. NAC reduced JNK activity by 60%, and MnTBAP reduced it by 90%. (C) JNK activity was also determined in HUVECs infected with either Ad-lacZ or Ad-DNp67 and exposed to vehicle or TNF. Ad-lacZ had no effect on TNF-induced JNK activity, whereas Ad-DNp67 reduced JNK activity by 55%.
Effect of antioxidants and dominant-negative p67phox on TNF-induced JNK activation. (A) Time-course of JNK activation was first determined by exposing HUVECs to TNF for up to 30 minutes and probing cell lysates for phospho-JNK (p-cJun) and total JNK (total-cJun). JNK activation was apparent within 5 minutes and was maximal by 15 minutes after TNF exposure. (B) HUVEC monolayers exposed to TNF in the presence of NAC or MnTBAP were lysed and examined for JNK activity using a functional JNK assay. NAC reduced JNK activity by 60%, and MnTBAP reduced it by 90%. (C) JNK activity was also determined in HUVECs infected with either Ad-lacZ or Ad-DNp67 and exposed to vehicle or TNF. Ad-lacZ had no effect on TNF-induced JNK activity, whereas Ad-DNp67 reduced JNK activity by 55%.
JNK inhibition prevents VE-cadherin phosphorylation, redistribution, and endothelial dysfunction
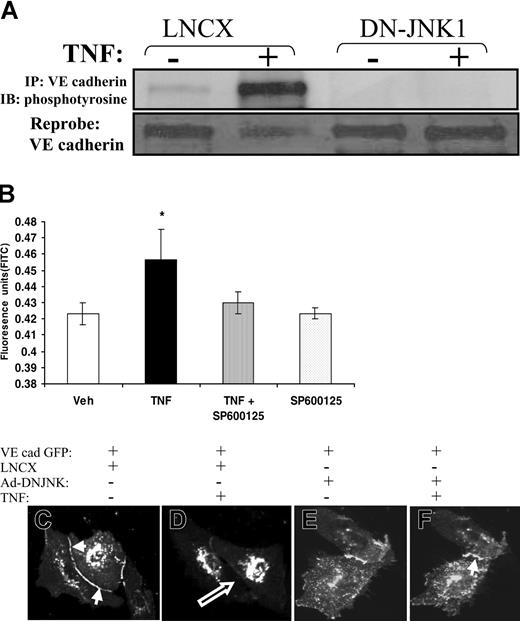
We examined the effect of dominant-negative JNK on TNF-induced VE-cadherin phosphorylation. Endothelial cells were transfected with the vector LNCX or with dominant-negative JNK before TNF exposure. In cells transfected with LNCX, TNF caused a 15-fold increase in VE-cadherin phosphorylation compared with vehicle controls. In contrast, dominant-negative JNK abolished TNF-induced VE-cadherin phosphorylation (Figure 6A).
Effect of JNK inhibition on VE-cadherin phosphorylation, intercellular gap formation and monolayer permeability. (A) TNF-induced tyrosine phosphorylation of VE-cadherin was examined after transfection with vector, LNCX (control), or DN-JNK1. The dominant-negative JNK mutant prevented TNF-induced tyrosine phosphorylation of VE-cadherin (top panel). (B) Confluent HUVEC monolayers were exposed to vehicle or saline in the presence or absence of the JNK inhibitor SP600125. The JNK inhibitor prevented TNF-induced FITC–dextran transmonolayer flux. *P < .05; n > 6 per group. (C-F) Dominant-negative JNK1 prevents TNF-induced junctional disassembly, intercellular gap formation, and VE-cadherin redistribution. HUVECs were cotransfected with full-length VE-cadherin GFP and either LNCX (C-D) or dominant-negative JNK1 (E-F) and were examined by confocal microscopy in the presence of vehicle (C, E) or TNF (D, F). As did nontransfected cells, VE-cadherin GFP localized to adherens junctions (C, solid arrow). In cells transfected with LNCX, TNF caused junctional disassembly and intercellular gap formation (D, open arrow). This was accompanied by redistribution of VE-cadherin GFP from adherens junctions. Transfection with dominant-negative JNK1 prevented TNF-induced junctional disassembly, gap formation, and VE-cadherin redistribution (F). This effect of dominant-negative JNK1 was present for up to 30 minutes of TNF exposure.
Effect of JNK inhibition on VE-cadherin phosphorylation, intercellular gap formation and monolayer permeability. (A) TNF-induced tyrosine phosphorylation of VE-cadherin was examined after transfection with vector, LNCX (control), or DN-JNK1. The dominant-negative JNK mutant prevented TNF-induced tyrosine phosphorylation of VE-cadherin (top panel). (B) Confluent HUVEC monolayers were exposed to vehicle or saline in the presence or absence of the JNK inhibitor SP600125. The JNK inhibitor prevented TNF-induced FITC–dextran transmonolayer flux. *P < .05; n > 6 per group. (C-F) Dominant-negative JNK1 prevents TNF-induced junctional disassembly, intercellular gap formation, and VE-cadherin redistribution. HUVECs were cotransfected with full-length VE-cadherin GFP and either LNCX (C-D) or dominant-negative JNK1 (E-F) and were examined by confocal microscopy in the presence of vehicle (C, E) or TNF (D, F). As did nontransfected cells, VE-cadherin GFP localized to adherens junctions (C, solid arrow). In cells transfected with LNCX, TNF caused junctional disassembly and intercellular gap formation (D, open arrow). This was accompanied by redistribution of VE-cadherin GFP from adherens junctions. Transfection with dominant-negative JNK1 prevented TNF-induced junctional disassembly, gap formation, and VE-cadherin redistribution (F). This effect of dominant-negative JNK1 was present for up to 30 minutes of TNF exposure.
To determine the effect of TNF-induced JNK activity on VE-cadherin redistribution and intercellular gap formation, HUVECs were cotransfected with VE-cadherin GFP and either LNCX or dominant-negative JNK and were exposed to vehicle control or TNF. Cells transfected with LNCX demonstrated rapid intercellular gap formation and redistribution of VE-cadherin from the intercellular junction, as did nontransfected cells. This separation was initiated in the center of cell-cell junctions and progressed to the lateral aspect of the junctions (Figure 6D). However, transfection with dominant-negative JNK prevented the disassembly of cell-cell junctions and intercellular gap formation for up to 30 minutes (Figure 6E-F).
To determine the functional effects of JNK inhibition on TNF-induced endothelial (EC) monolayer dysfunction, we examined transmonolayer permeability using FITC-labeled albumin in the presence or absence of the JNK inhibitor, SP600125. As expected, TNF caused a 50% increase in albumin transit across the monolayer, whereas SP600125 prevented this phenomenon. SP600125 alone had no effect on albumin transit (Figure 6B).
TNF activates PAK1 upstream of JNK and VE-cadherin phosphorylation
We previously demonstrated that PAK1 acts upstream of p47phox phosphorylation and oxidase activation and that TNF activates endothelial cell PAK1.20,21 As further evidence that the NADPH oxidase and JNK participate in junctional disassembly, we blocked PAK1 activation with the kinase-dead mutant PAK1(K298A). Ad-PAK1(K298A) markedly reduced TNF-induced JNK activity compared with controls (Figure 7A). In parallel, Ad-PAK1(K298A) prevented TNF-induced VE-cadherin and α-catenin phosphorylation (Figure 7B). Coinfection of HUVECs with Ad-PAK1(K298A) and Ad-VE-cadherin GFP demonstrated that Ad-PAK1(K298A) prevented TNF-induced intercellular gap formation and cell-cell separation (data not shown).
Effect of AdPAK1(K298A) on JNK and VE cadherin. (A) Kinase-inactive PAK1, AdPAK1(K298A), reduces TNF-induced JNK activity and junctional protein phosphorylation. TNF-induced JNK activation was examined in cells infected with either Ad-lacZ (control) or Ad-PAK1(K298A). In cells infected with Ad-lacZ, TNF caused robust JNK activity, similar to that in noninfected cells. In contrast, Ad-PAK1(K298A) markedly reduced TNF-induced JNK activation. (B) Tyrosine phosphorylation of VE-cadherin and α-catenin was similarly examined in cells infected with Ad-lacZ or AdPAK1(K298A). The PAK1 mutant also reduced tyrosine phosphorylation of VE-cadherin and α-catenin. (C) Histograms illustrate the densitometry values for VE-cadherin (left) and α-catenin (right) phosphorylation. (D) Schematic illustrates the potential signaling pathway linking TNF-induced endothelial oxidant production and junctional phosphorylation.
Effect of AdPAK1(K298A) on JNK and VE cadherin. (A) Kinase-inactive PAK1, AdPAK1(K298A), reduces TNF-induced JNK activity and junctional protein phosphorylation. TNF-induced JNK activation was examined in cells infected with either Ad-lacZ (control) or Ad-PAK1(K298A). In cells infected with Ad-lacZ, TNF caused robust JNK activity, similar to that in noninfected cells. In contrast, Ad-PAK1(K298A) markedly reduced TNF-induced JNK activation. (B) Tyrosine phosphorylation of VE-cadherin and α-catenin was similarly examined in cells infected with Ad-lacZ or AdPAK1(K298A). The PAK1 mutant also reduced tyrosine phosphorylation of VE-cadherin and α-catenin. (C) Histograms illustrate the densitometry values for VE-cadherin (left) and α-catenin (right) phosphorylation. (D) Schematic illustrates the potential signaling pathway linking TNF-induced endothelial oxidant production and junctional phosphorylation.
Discussion
We previously demonstrated that TNF-induced endothelial dysfunction is associated with intercellular gap formation, a process regulated by tyrosine phosphorylation of VE-cadherin.11 Here we provide evidence that TNF-induced VE-cadherin phosphorylation is mediated by intracellular oxidants specifically generated by the endothelial NADPH oxidase, and we link oxidant production with JNK activation.
We found that oxidants are produced in vascular endothelium early after TNF exposure, supporting the hypothesis that such oxidants act proximally in the signaling pathways leading to endothelial dysfunction. The transient nature and low levels of intracellular oxidant production, as well as its localization to membrane sites, suggests that oxidant-mediated junctional protein phosphorylation may require proximity of oxidant production to downstream targets. In contrast, persistent and widespread intracellular oxidant production could potentially activate unnecessary signaling pathways. We also found that 2 biochemically distinct antioxidants, NAC and MnTBAP, prevented TNF-induced decreases in TER, implicating endogenously produced oxidants during junctional disassembly. In support of this inference, we found that NAC and MnTBAP prevented TNF-induced tyrosine phosphorylation of VE-cadherin. Oxidants have been shown to cause phosphorylation of intracellular proteins.22 However, intracellular oxidant production has not been previously linked with VE-cadherin phosphorylation during cytokine exposure. Taken together with the effect of antioxidants on TNF-induced decreased TER, these findings suggest that TNF-induced EC barrier dysfunction is oxidant mediated and occurs through the regulation of VE-cadherin phosphorylation.
Although intracellular oxidant production has been demonstrated during TNF exposure, the source of these oxidants is unclear. A phagocyte-type NADPH oxidase is expressed by vascular endothelium3,5,23,24 and has been implicated in the endothelial response to TNF,2,19 VEGF,21 HIV Tat,13 and angiotensin II.25 Endothelial cells appear to generate oxidants from multiple sources, depending on the cell type and agonist. Hypoxia, reoxygenation, and shear stress all increase endothelial oxidant production by different pathways.26-29 Recent evidence suggests that the NADPH oxidase may be an important pathway during the response to TNF.2,19 The present study specifically implicates NADPH oxidase as a primary oxidant–generating pathway responsible for TNF-induced endothelial barrier dysfunction. This is supported by the findings that DPI abolished TNF-induced oxidant production and that p67(V204A) prevented TNF-induced VE-cadherin phosphorylation. In addition, these findings confer specificity to the effects of the antioxidants because a recent study suggests that thiol antioxidants such as NAC may decrease TNF signaling independent of antioxidant function.30
Junctional protein phosphorylation is thought to be a potential mechanism for regulating the stability of adherens junctions and barrier dysfunction.31-33 For instance, our laboratory and others have shown that intercellular gap formation in endothelial monolayers is dependent on phosphorylation events at the adherens junction.11,16,17 Thus, the parallel tyrosine phosphorylation of α-catenin and VE-cadherin by TNF represents a biochemical correlate of junctional dissociation and intercellular gap formation. However, the mechanism underlying junctional protein phosphorylation is undetermined. Oxidant-dependent intracellular phosphorylation events are important in growth factor receptor activation and epithelial barrier function,22,34-36 but few studies have examined the response of individual junctional components to cytokine-induced oxidant activation. Our findings suggest that phosphorylation of VE-cadherin, the predominant component of endothelial adherens junctions,37-40 proceeds through the production of endogenous oxidants. Although the mechanism of oxidant-mediated tyrosine phosphorylation of VE-cadherin is unclear, oxidants are known to reversibly inactivate tyrosine phosphatases.41,42 Thus, local inactivation of such phosphatases at lateral membrane sites may facilitate tyrosine phosphorylation of VE-cadherin. A potential target may be vascular endothelial protein tyrosine phosphatase (VE-PTP), a junctional phosphatase associated with, and capable of dephosphorylating, VE-cadherin.43 Our observation that TNF-induced phosphorylation of α-catenin parallels VE-cadherin phosphorylation and is similarly decreased by p67(V204A) further suggests that oxidant-mediated dephosphorylation events may be specifically targeted to junction proteins.
Surprisingly, we found that JNK may also participate in the phosphorylation of junctional proteins. Dominant-negative JNK abolished TNF-induced VE-cadherin phosphorylation and stabilized cell-cell junctions, whereas the JNK inhibitor SP600125 prevented TNF-induced EC barrier dysfunction. These findings suggest that the effects of JNK on EC dysfunction are mediated through junctional protein phosphorylation and dissociation of adherens junctions. Further, both antioxidants and p67(V204A) decreased TNF-induced JNK activation. Thus, NADPH oxidase–derived oxidants appear to mediate VE-cadherin phosphorylation at least in part through JNK activation. Although the mechanism of JNK effect on VE-cadherin is unclear, JNK may lie immediately upstream of a kinase that phosphorylates VE-cadherin or, alternatively, may direct the effects of the NADPH oxidase to the lateral junction microenvironment.
Recently, we implicated the MAP4 kinase p21-activated kinase (PAK1) in the activation of the endothelial NADPH oxidase by VEGF and HIV Tat, presumably acting upstream of p47phox phosphorylation.20,21 We therefore hypothesized that PAK1, which is known to be rapidly activated by TNF,20 may also regulate VE-cadherin phosphorylation and junctional behavior. Our finding that PAK1(K298A) prevented TNF-induced VE-cadherin, α-catenin phosphorylation, and gap formation supports a critical role for PAK1 activity in the junctional protein response to TNF. In addition, the suppression of JNK activation by PAK1(K298A) is consistent with the participation of PAK1 at a proximal level. Although we speculate that PAK1 may impart its effects through activation of the NADPH oxidase, PAK1 is likely to have multiple downstream effectors, many of which may be unrelated to the oxidase. Interestingly, re-expression of VE-cadherin in VE-cadherin null cells causes redistribution of Rac1 and PAK1 to the membrane/cytoskeletal fraction, suggesting reciprocal regulation of VE-cadherin and PAK1 function.44
We have demonstrated that TNF signaling during endothelial barrier dysfunction requires the production of intracellular oxidants. Such oxidant production is transient, and the primary source appears to be NADPH oxidase. Intracellular oxidants cause intercellular gap formation by phosphorylation of junctional proteins. Elucidation of the mechanisms by which oxidants phosphorylate junctional proteins may provide another target for the modulation of endothelial activation during inflammatory states.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-05-1868.
Supported in part by the Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program), the National Institutes of Health (RO1 GM067674-01A1, R01-HL61897, R01-HL67256), and the Veterans Administration Research Service.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.