Abstract
BACKGROUND: Classic strategies for peripheral blood progenitor cell (PBPC) mobilization include daily administration of a growth factor, such as filgrastim, alone or with marrow suppressive chemotherapy. A single injection of pegfilgrastim has been shown to be comparable to daily injections of filgrastim in the treatment of chemotherapy-induced neutropenia. One of the objectives of this phase 1–2 study was to provide dose-finding information regarding the efficacy and kinetics of cytokine alone PBPC mobilization with pegfilgrastim.
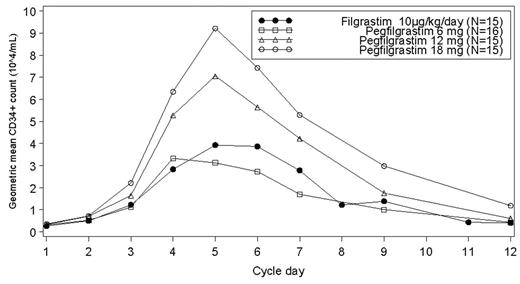
METHODS: Chemotherapy naïve subjects with a variety of solid tumors were randomized to receive a single administration of 6, 12 or 18 mg of pegfilgrastim on day 1 or daily administration of 10 μg/kg filgrastim from day 1 until day 7. Daily blood samples for peripheral CD34+ analysis were collected from days 1 to 7 and then on days 9 and 12. No chemotherapy was administered during this cycle. For each subject, peak CD34+ cell count was defined as the maximum cell count observed between days 3 to 7, inclusive.
RESULTS: Of 61 subjects randomised into the study, all received study drug (15 subjects each in the pegfilgrastim 12mg, 18mg and filgrastim groups and 16 subjects in the pegfilgrastim 6mg group). The treatment groups were balanced in terms of demographics and baseline characteristics. The most common tumor types were non-small cell lung cancer (n= 23 [38%]) and ovarian (n= 19 [31%]). The mean peak CD34+ cell count was similar in the filgrastim and pegfilgrastim 6mg groups (4.51 and 4.24 x 104/mL respectively) whereas the pegfilgrastim 12mg and 18mg groups had higher mean peaks (8.18 and 9.96 x 104/mL respectively). Pegfilgrastim 12mg and 18mg mobilized significantly more peripheral CD34+ cells than filgrastim (p= 0.025 and 0.003 respectively). The subject incidence of SAEs was low and comparable between groups.
CONCLUSION: In chemotherapy naïve subjects with solid tumor, single day administration of pegfilgrastim is at least as efficacious as repeated daily injections of filgrastim (10 μg/kg) in its ability to successfully mobilize peripheral CD34+ cells. A dose response relationship of CD34+ mobilization is observed. Pegfilgrastim 12mg and 18mg mobilize higher numbers of CD34+ cells than daily administrations of filgrastim. Pegfilgrastim at 6, 12 and 18mg is generally safe and well tolerated in this setting.
Author notes
Corresponding author