Abstract
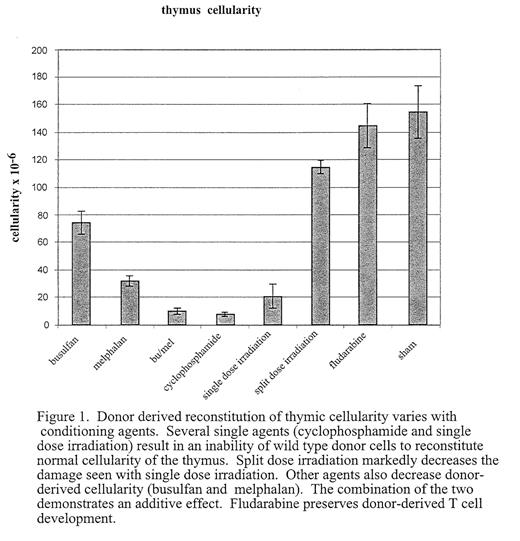
A key component of long-term outcome after stem cell transplant (SCT) is successful reconstitution of the immune system. Effective reconstitution of antigen-specific T-cell immunity requires de novo T cell generation. Bone marrow derived progenitors seed the thymus and undergo a complex process involving lineage commitment, proliferation and selection. Coordinated interaction of marrow-derived lymphoid progenitors with thymic stromal cells is required for successful T lymphopoiesis in the post-natal thymus. Disruption of the microenvironment can result in disrupted T cell lymphopoiesis. One cause of prolonged defects in generating functional T lymphocytes after BMT is damage to the thymic microenvironment induced by radiation or cytotoxic therapy. However, the impact of individual agents, administered at myeloablative or non-myeloablative doses, on the thymic microenvironment has not been fully evaluated. In addition, mechanisms by which stromal injury modifies T cell production and maturation have only begun to be understood. We have developed a model system using immunodeficient mice as a platform on which to assess thymic reconstitution. The thymus of mice deficient for the alpha chain of the IL-7 receptor (IL7R−/−) is relatively depleted of lymphoid cells and can be reconstituted following transplant of wild type marrow administered without myeloablative or immunosuppressive treatment. Injection of low doses of wild type bone marrow into these mice results in low levels of marrow chimerism and a normally cellular thymus repopulated with donor-derived lymphocytes. The ability to achieve this reconstitution appears to depend on absolute numbers of early intra-thymic precursors, rather than on total thymic cellularity. We have exploited this model to differentially assess the effects of cytotoxic agents including radiation and immunosuppressive drugs, on the capacity of the thymic microenvironment to support the maturation of normal lympoid progenitors (Figure 1). We demonstrate that some agents do not affect the ability of the thymic microenvironment to support reconstitution (eg fludarabine), others nearly ablate it (cyclophosphamide). We are also able to show dose, schedule, and synergistic effects on the ability of the thymic microenvironment to support de novo T cell lymphopoeisis. Distinct morphologic and phenotypic effects can be demonstrated by different agents (eg busulfan versus thiotepa) with preliminary data suggesting that the effects are mediated by injury to different stromal subsets. It is anticipated that this information will lead to strategies to both minimize delayed immune reconstitution and to augment T cell lymphopoiesis post-transplant. In addition, further evaluation of impaired thymic reconstitution will augment the understanding of lymphostromal interactions crucial to normal T cell lymphopoiesis.
Author notes
Corresponding author