Abstract
Objectives. Corticosteroid-resistant acute GvHD remains extremely difficult to manage and is associated with very high morbidity and mortality. There are many drugs and therapeutic procedures used as the second or third line treatment, e.g. ATG, several monoclonal antibodies, mycophenolate mofetil, tacrolimus, sirolimus (rapamycin), photopheresis, pentostatin, and denileukin diftitox. We hypothesized that there are several logical reasons also for the usage of pulse CY in the treatment of GvHD: (1) the dismal prognosis of patients with acute GvHD non-responding to the steroid treatment; (2) the established efficacy of CY for the treatment of many autoimmune disorders and for immunosuppression before allogeneic hematopoietic stem cell transplantation; (3) the described efficacy of high-dose CY (200 mg/kg) supported by autologous or pseudoautologous hematopoietic stem cell transplantation for the treatment of GvHD. Here we will report on the results of the treatment of steroid-resistant GvHD with pulse CY in 15 consecutive patients.
Methods. In an established GvHD requiring therapy, the first option was usually prednisone or methylprednisolone at the dose of 2 mg/kg/day together with cyclosporine A. For GvHD progression or unresponsiveness, despite initial treatment, CY was administered at the dose of about 1000 mg/m2 as an iv. infusion under vigorous hydration. Mesna and/or growth factors were not routinely used. Continuous administration of cyclosporine A was not interrupted during the CY treatment.
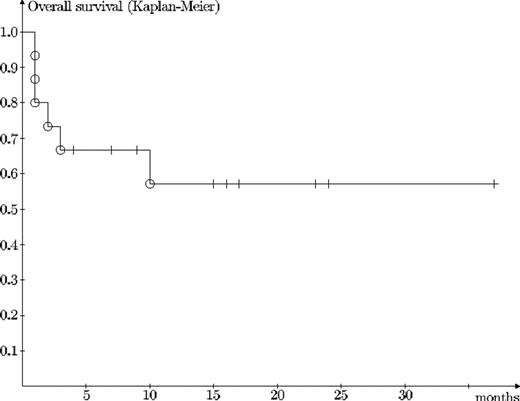
Results. Pulse CY (17 administrations in total) showed high effectivity in the treatment of GvHD of the skin (100 % response rate, RR), oral cavity (100 % RR), and the liver (70 % RR with CY alone, 80 % with additional therapy). However, its ability to control severe intestinal GvHD was much lower (0 % RR with CY alone, 29 % RR with additional therapy, Fisher exact probability p<0,007). Pulse CY, except for manageable myelosuppression of short duration (usually 1–4 days) in some patients, exerts a remarkably good toxicity profile and does not appear to increase the risk of opportunistic infections, mixed chimerism, relapses, or posttransplant lymphoproliferative disease. Leukopenia with WBC below 1.109/L developed after 9 out of the 17 CY administrations (53 %) and thrombocytopenia under 50.109/L developed six-times (35 %). It is interesting to note that the response to Cy pulse did not significantly depend on the development of critical leukopenia (Fisher exact probability p=0.6). Six patients died. In five of them the direct cause of death was therapy-refractory intestinal GvHD and one patient died from recurrent AML. Overall survival is 57 % with longest follow-up of 37 months.
Conclusions. Pulse CY seems to be cheep, effective and non-toxic treatment of steroid-refractory GvHD of the skin and liver and produces long-term control of this posttransplant complication. Its ability to control intestinal GvHD is much lower, however. In future trials, this pulse CY might also be tested in combination with other drugs with a different mechanism of action. Acknowledgments. Supported by a Research Grant of the Ministry of Health of the Czech Republic No: MZ 00065269705.
Author notes
Corresponding author