Abstract
Introduction: In patients undergoing total knee replacement (TKR), the post-operative period is a time of intense thrombotic stimulus often resulting in venous thromboembolism (VTE), precisely when surgical hemorrhage must be minimized: anticoagulant management requires a delicate balance. Ximelagatran (Exanta®, AstraZeneca), the first oral alternative to warfarin, has a rapid onset of action and requires no coagulation monitoring or dose adjustment. Three randomized, double-blind trials of ximelagatran vs. warfarin as VTE prophylaxis after TKR were completed: Study 236 (n=680), EXULT A (n=2301) and EXULT B (n=2303). Ximelagatran 36 mg BID provided efficacy superior to well-controlled warfarin (target INR 2.5) administered for 7–12 days, while 24 mg BID yielded numerically better but not statistically superior efficacy; neither dose increased bleeding.
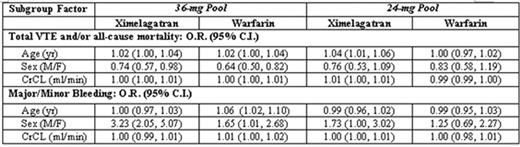
Objective: We performed pooled subgroup analyses to assess pre-specified subgroup factors with regard to thromboembolic and adjudicated major/minor bleeding events.
Methods: Two comparison pools were created: 36-mg pool (ximelagatran 36 mg and corresponding warfarin groups from EXULT A and B) and 24-mg pool (ximelagatran 24 mg and corresponding warfarin groups from Study 236 and EXULT A). Prognostic factors were examined using multiple logistic regression analysis. Treatment by factor interactions are reported.
Results: In the 36-mg pool, no significant treatment interactions were observed for efficacy (i.e., the influence of each subgroup factor on ximelagatran and warfarin patients was similar). However, in the 24-mg pool, increases in age and CrCL indicated a disproportionate increase in the composite efficacy endpoint of total VTE and/or all-cause mortality for the 24-mg group compared to the warfarin group. Subgroup analyses of major/minor bleeding events showed no significant treatment interactions in the 24-mg pool. In the 36-mg pool, increases in age resulted in increased bleeding in the warfarin group compared to the 36-mg group. Men on ximelagatran 36 mg exhibited a greater risk of bleeding than did men on warfarin. No factors were identified to explain the observed increase in risk.
Conclusions: Subgroup analysis results for the ximelagatran 36-mg and 24-mg pools were generally consistent with those in the overall population, with the incidence of the composite efficacy endpoint consistently lower in the ximelagatran groups than in the warfarin group for most pre-specified subgroups. Patients expected to have higher ximelagatran exposure due to renal impairment and increased age showed no significant difference in bleeding risk compared with warfarin. This analysis supports a 36-mg dose for all patients undergoing TKR.
Author notes
Corresponding author