Abstract
Introduction
Antisense oligonucleotides are used to inhibit messenger RNA function and inhibit protein translation. Bcl-2 expression proteins have been implicated in mediating resistance to apoptotic cell death in Waldenstrom Macroglobulinemia (WM). Oblimersen sodium is a specific inhibitor of Bcl-2 expression and has shown activity in multiple myeloma and in Waldenstrom cell lines. The in vitro data led to the development of a phase I study for WM patients with relapsed or refractory disease.
Materials and Methods
Eligible patients had symptomatic WM who had failed prior cytotoxic chemotherapy. All patients had to have one of the following: a hemoglobin <11 g/dL, a platelet count <100,000/uL, bulky lymphadenopathy, or hyperviscosity syndrome. As a phase I study, the primary end point was toxicity assessment, but all patients were assessed for response, using monoclonal protein levels. Patients were enrolled and evaluated in cohorts of three. Drug was administered as a 24-hour continuous infusion for 7 days every 21 days, with intrapatient dose escalation each subsequent cycle beginning at 3 mg/kg/day up to a maximum of 7 mg/kg/day days 1–7 for a maximum of 8 cycles.
Results
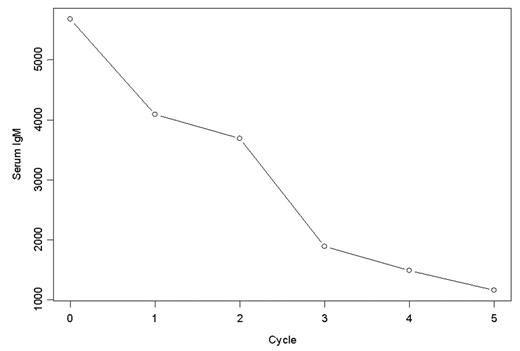
To date 9 patients have been accrued, six at dose level one and three patients at dose level two. Data is available for the first six entered. Five women and 1 man have been accrued with a median age of 74.5 years, ranging from 58 to 81. Four patients had relapsed from prior therapy, 2 were refractory. Three had a history of transfusions. The median number of prior regimens was 4 (range 2–7). One patient had a dose-limiting toxicity on the first cycle of therapy, which was grade 3 fatigue and anorexia, and the dose for this patient was reduced. Two patients had non-hematologic grade 3 toxicity, possibly related to therapy. Five out of six patients on dose level one had grade 3 or greater hematologic toxicity. These toxicities occurred after cycle 1 was completed, during the toxicity observation period and were not considered dose limiting toxicities influencing dose escalation. Three patients required dose reductions in subsequent cycles. To date one patient has had a partial response with her serum M spike falling from 3.6 to 1.1 g/dL. Her quantitative IgM fell from 6,000 mg/dL to 1,000 mg/dL(figure).
Conclusion
Oblimersen was well tolerated at the first dose level in patients with WM. Hematologic toxicity is frequent in this cohort with heavily involved bone marrows when the dose was escalated during successive cycles. Dose reductions with subsequent cycles were frequently required. Evaluation of the maximum tolerated dose continues. Data on response for this dose level is promising, where efficacy will be fully evaluated in the phase II portion of this trial.
Author notes
Corresponding author