Abstract
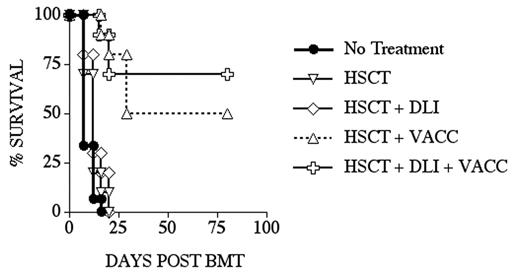
Allogeneic HSCT is an important therapy with curative potential for a variety of malignant diseases, including leukemias, lymphomas and some solid tumors. Despite significant progress in reducing treatment-related mortality, malignant relapse remains a major problem. We are developing DNA vaccines that encode gene products closely related to self-antigens, including xenogeneic DNA and mutated DNA, and have initiated clinical trials of DNA vaccines in patients with advanced melanoma or prostate cancer. Using the B16 mouse melanoma model, we have shown that immunization with human TRP-2 DNA (xenogeneic melanoma differentiation antigen - MDA) or Opt-Tyrp1 DNA (a mutated MDA related to TRP-2, which we have optimized for CD8 epitopes), can induce tumor protection, including against established tumors. We hypothesized that immunization of allogeneic HSCT recipients (or their donors) against specific tumor antigens could decrease the risk of relapse without enhancing graft-versus-host disease (GVHD). In an MHC-matched minor antigen-mismatched mouse HSCT model (LP into B6), we found that: (1) by day 28 after transplant, recipients of an allogeneic T-cell depleted (TCD)-HSCT have considerable numbers of splenic T cells, including de novo generated donor T cells, which suggests that vaccination aimed at T cells might be feasible; (2) post-HSCT DNA immunization against a single tumor antigen can provide protection from a tumor challenge that is comparable to that observed with a whole cell vaccine (B16-GM-CSF) and significantly greater than HSCT alone; (3) DNA immunization post-HSCT can induce tumor-specific CD8+ T cells of donor origin (detected by ELISPOT or intracellular cytokine flow cytometry assay); (4) the combination of donor leukocyte infusion (DLI) and post-HSCT DNA immunization further enhances tumor-free survival (Figure); (5) there is no evidence of GVHD in multiple experiments using a clinical GVHD score to monitor recipients; and (6) the effects of post-HSCT DNA immunization on both tumor-free survival and CD8+ T cell responses have been validated for two different DNA vaccine strategies (hTRP-2 + GM-CSF DNA, or Opt-Tyrp1 DNA). These results demonstrate that DNA immunization after allogeneic TCD-HSCT can induce potent anti-tumor effects without the induction of GVHD. This and similar investigations provide a strong rationale for the development of novel therapeutic strategies that combine allogeneic HSCT, post-transplant tumor vaccination and adoptive cell therapy in human clinical trials.
Author notes
Corresponding author