Abstract
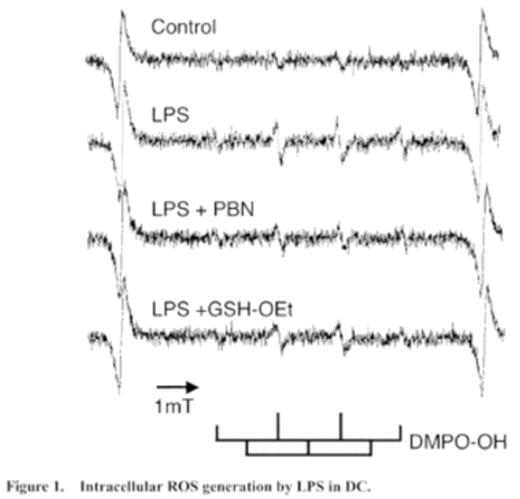
The role of reactive oxygen species (ROS) and redox status on the maturation of dendritic cells (DC) was examined using alpha-phenyl-tert-butylnitrone (PBN) and glutathione reduced form ethyl ester (GSH-OEt). PBN is widely used as a spin trapping agent in electron paramagnetic resonance (EPR) studies. Since PBN reacts with free radical species and stabilizes them, PBN serves as an antioxidant. GSH not only serves as a major antioxidant but also plays a central role in maintaining intracellular redox balance, and GSH-OEt increased the intracellular GSH level. When human monocyte-derived DC were stimulated with LPS, up-regulation of the expression of the surface molecules (HLA-DR, CD40, CD80, CD86 and CD83), production of cytokines (TNF-α and IL-12p70) and allostimulatory capacity were observed. The LPS-induced cytokine production was suppressed by both PBN and GSH-OEt, while the up-regulation of the expression of surface molecules and the allostimulatory capacity were suppressed by only GSH-OEt but not by PBN. The mean values of TNF-α of LPS, LPS+PBN and LPS+GSH-OEt were 1220, 783 and 238 pg/ml, respectively (n=12). Those of IL-12p70 were 632, 358 and 5 pg/ml, respectively (n=12). The EPR study revealed that ROS was generated by LPS and that the ROS generation was attenuated by both PBN and GSH-OEt. Flow cytometric analysis revealed that intracellular GSH content was decreased by LPS and that this reduction was attenuated by GSH-OEt not by PBN. That is, PBN quenched the generated ROS but did not affect the redox status, while GSH-OEt not only quenched the generated ROS but also affect the redox status in the LPS-stimulated DC. The allostimulatory capacity of DC correlates with the expression of surface molecules and it does not depend on IL-12 production. Therefore, our findings suggest that ROS and redox status have distinctive effects on the maturation of DC. The ROS is involved in the cytokine production, while the redox status is involved in the up-regulation of surface molecules and allostimulatory capacity in the LPS-stimulated DC. LPS induces the activation of intracellular signaling cascade via Toll-like receptor 4. The signaling cascade is consist of two distinct pathways, MyD88-dependent and independent pathways. In DC, the MyD88-dependent pathway is involved in the cytokine production, while the MyD88-independent pathway is involved in the up-regulation of CD80 and CD86. The former may be ROS-sensitive, the latter may be redox status-sensitive. Differential regulation of the ROS and the redox status in DC may be possible to adequately modulate inflammatory and immune responses in the disease, such as graft-versus-host disease.
Author notes
Corresponding author