Abstract
Introduction:
Features of multiple myeloma (MM) include a proliferative clonal plasma cell population, bone resorption, and neovascularization. Cytokines and chemokines represent two families of molecules that are capable of propagating and enhancing these disease features. In this study, we have utilized antibody array technology to assess the contributions of cytokines and chemokines to the progression of disease, and evaluated the contribution of stromal cells (SCs) in their production.
Methods:
Wild-type and IL-1beta transduced KAS 6/1 myeloma cell lines or bone marrow cells isolated from patients with monoclonal gammopathy of undetermined significance (MGUS), smoldering myeloma (SMM), and MM were cultured for 48 hrs. Culture supernatants were either analyzed directly, or co-cultured with normal SCs for an additional 48 hrs +/− IL-1 inhibitors, after which the supernatants were removed and analyzed using antibody arrays. SCs alone were also cultured with recombinant IL-1beta +/− IL-1 receptor antagonist (IL-1Ra) to define IL-1 dependent effects. IL-6 and IL-8 ELISAs were utilized to quantify IL-6 and IL-8 levels, and validate antibody array findings.
Results:
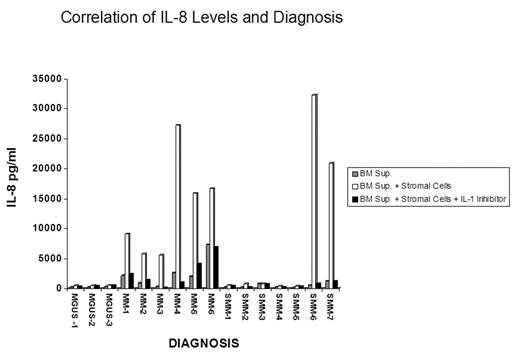
Antibody array analysis of IL-1 effects on stromal cell cultures using recombinant IL-1beta and supernatants from an IL-1beta transduced myeloma cell line demonstrated that stimulation of IL-6, MCP-1 and IL-8 were induced in an IL-1 and stromal cell dependent manner. Although levels of TIMP-2 varied in these cultures, they appeared unrelated to an IL-1 effect. Studies utilizing supernatants from patient bone marrow cells co-cultured with SCs resulted in levels of IL-6, MCP-1, and IL-8 higher than those seen with patient supernatants alone or SC cultures alone. More interestingly, the IL-8 levels appeared to correlate with diagnosis; MGUS samples generated low levels and MM samples stimulated high levels. Furthermore, this stimulation was reduced by the addition of IL-1 inhibitors, demonstrating a dependence on IL-1. To confirm the relationship between diagnosis and IL-8 production, the levels of IL-8 produced by the bone marrow supernatants were quantified directly by ELISA. Correlating with the antibody array data, background production of IL-8 from the cultures of patient cells alone was lower than the corresponding co-culture value. Supernatants from MM patients and a subset of SMM patients stimulated high levels of SC IL-8 secretion in contrast to bone marrow cell supernatants from MGUS patients and most SMM patients. This activity was inhibited by IL-1 inhibitors (see Figure). The IL-8 levels closely parallel the IL-1beta induced IL-6 levels in the same samples.
Conclusion:
These data indicate that the concentration of IL-8 may be relevant to the pathogenesis of MM. IL-8 production is largely dependent on SCs, and production appears to be at least partially dependent on IL-1 function. IL-8 is a chemokine with activities including chemotaxis of neutrophils, increased vascular permeability and angiogenesis. IL-8 expression has been implicated in multiple tumor types and may play an important role in the stimulation of angiogenesis during the progression from MGUS to active MM.
Author notes
Corresponding author