Abstract
Background: Although thousands of patients (Pts) receive chemotherapy every year in the United States, only few retrospective studies have been done to address the risk factors for chemotherapy (CT) induced neutropenia in patients with cancer. The data in androgen-independent prostate cancer (AIPC) patients is extremely scarce. We decided to perform a retrospective analysis of patients with metastatic AIPC treated with chemotherapy in an open phase II clinical trial looking for predictive factors for neutropenia.
Methods: We reviewed the records of all patients with AIPC who completed at least 2 cycles of CT with cyclophosphamide 50 mg/m2, etoposide 50 mg/m2 and estramustine 280 mg orally every night for 14 days out of a 28 day-cycle. We divided the patients into 2 groups according to neutropenia grade 3–4 (Group 1) versus 0–2 (Group 2). Risk factors reviewed included age, number of organs involved with metastases, number of bone metastasis, prior radiotherapy (RT) to the prostate/pelvis, prior number of areas treated with palliative RT, prior CT regimens, baseline PSA, absolute neutrophil count (ANC) and ECOG performance status (PS).
Results: See table and figures.
| Group . | 1 . | 2 . |
|---|---|---|
| Neutropenia Grade | 3–4 | 0–2 |
| # of Pts | 7 | 11 |
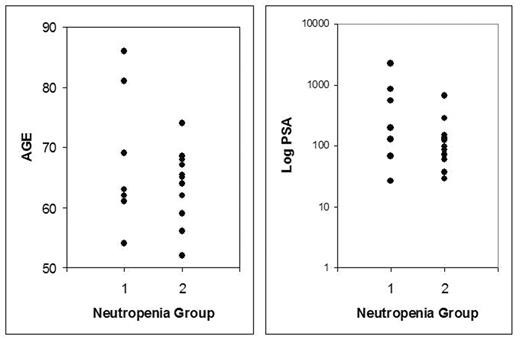
| Age | 68 (58–86) | 63 (52–74) |
| # organs involved | 2.1 | 1.6 |
| # bone metastases | 10.9 | 9.5 |
| RT prostate/pelvis | 0.1 | 0.4 |
| # palliative RT courses | 1 | 0.6 |
| # prior CT regimens | 0.4 | 0.2 |
| Baseline ANC | 3.6 | 3.8 |
| Baseline PS ECOG | 1.3 | 1.4 |
| Baseline PSA | 584 (26–2268) | 157 (37–65) |
| Group . | 1 . | 2 . |
|---|---|---|
| Neutropenia Grade | 3–4 | 0–2 |
| # of Pts | 7 | 11 |
| Age | 68 (58–86) | 63 (52–74) |
| # organs involved | 2.1 | 1.6 |
| # bone metastases | 10.9 | 9.5 |
| RT prostate/pelvis | 0.1 | 0.4 |
| # palliative RT courses | 1 | 0.6 |
| # prior CT regimens | 0.4 | 0.2 |
| Baseline ANC | 3.6 | 3.8 |
| Baseline PS ECOG | 1.3 | 1.4 |
| Baseline PSA | 584 (26–2268) | 157 (37–65) |
Conclusions: Average age and PSA were the only risk factors that were different between the patients who developed severe neutropenia and the ones who did not, albeit not statistically significant. Studies with a larger sample size may detect clinically and statistically significant differences.
Author notes
Corresponding author