Abstract
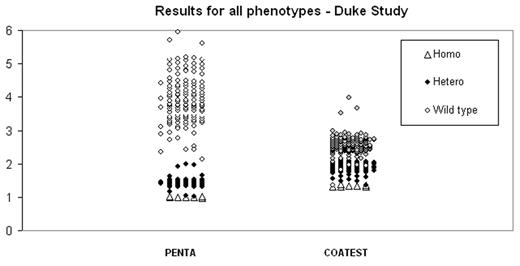
Activated protein C (APC) resistance is the most frequent hereditary defect associated with deep venous thrombosis. Major cause of APC resistance phenotype is due to a point mutation of factor V (Factor V Leiden). A new clotting assay, Pefakit® APC-R Factor V Leiden (Pentapharm Ltd., Switzerland) for the detection of APC resistance phenotype was evaluated at two tertiary care hospitals, Duke University Medical Center, USA (Duke) and University of Vienna, Austria (Vienna). Samples of 242 subjects from Duke and 187 subjects from Vienna were included in the study among patients who were subjects for thormbophilia screening. The Pefakit® method is based on clotting time measurement triggered by a prothrombin activator added to a mixture of patient plasma diluted with factor V deficient plasma with and without APC. Robustness and specificity of the assay is enhanced by elimination of possible disturbing influence by factors upstream the coagulation cascade and heparin interference is precluded up to heparin level of 2 IU/ml by heparin inhibitor added to the regent. A similar, FDA approved commercial APC-R kit (COATEST of IL) was used for method comparison. Patients with elevated factor VIII (n=11), Coumadin (n=23), Lupus Anticoagulant (n-14), protein C deficient (n=7), protein S deficient (n=9), AT III (n=6) and women with pregnancy (n=9) were included in Duke study and no interference were found in phenotype. Using PCR/FRET DNA method as reference method the Pefakit® method provided 100 % sensitivity and 100 % specificity for the Vienna study and 99.0 % sensitivity and 98.6 % specificity for the Duke study and the COATEST provided 97.1 % specificity and 93.2 % sensitivity with the Duke study. Using two levels of genotype controls both studies showed similar intra and inter-assay precision (less than 6 % for the Vienna study and 9 % for the Duke study) as compared with the gold standard IL APC-R COATEST kit (less than 5 % CV). Of great interest one false positive sample from the Duke study is under investigation due to that the functional detection of the assay is supposed to detect other FV mutations leading to APC-R phenotype as well. Reasons that cause the other two false negative results for the Duke study are still unknown and under investigation. Both studies showed that the Pefakit® is simple and rebust assay. Both wild type and heterozygous groups have much higher ratio as compared with the reference method in differentiating them from homozygous phenotype.
Author notes
Corresponding author