Abstract
Background: Proteome analysis is a direct measurement of proteins in terms of their presence and relative abundance in a defined system. The overall aim of a proteomic study is characterization of the complex network of cell regulation. Different states of a cell can be compared and specific qualitative and quantitative protein changes can be identified. We focused our first proteom-investigations on G-CSF mobilized CD34+ stem cells from peripheral blood (PB).
Methods: Mononuclear cells from healthy donors were isolated by a standard Ficoll-Hypaque gradient separation method after leucapheresis from PB. An Auto-MACS (Miltenyi) and FACS Vantage SE cell sorter (Becton Dickinson) was used to highly enrich (>99%) CD34+ cells fractions. Sample preparation, determination of protein concentrations, 2D-gel-electrophoresis with a 1D-separation isoelectric focusing (IEF) with immobilised pH gradient (IPG) strips (17cm), 2D-separation with SDS-PAGE were performed and described in Proteome Works System (BioRad), pH range from 3 until 10 and molecular weight from 5 until 200kDa. Sypro ruby stained gels were used for protein identification by peptide mass fingerprint analysis with matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS).
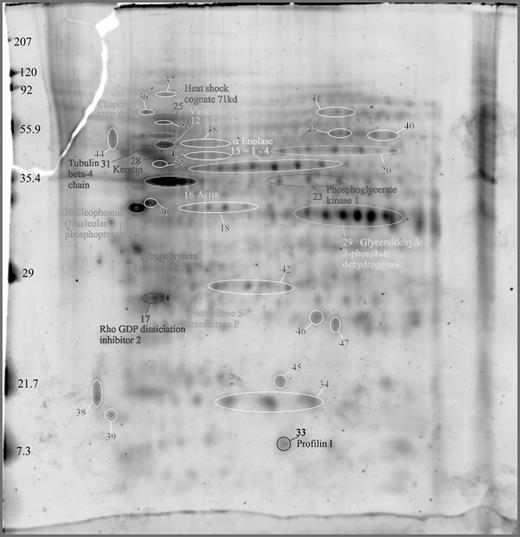
Results: A.) The pattern of the G-CSF mobilized CD34+ fraction from PB cells is complex and shows >1000 protein spots (dependent on protein concentration) in silver stain using PDQuest.
B.) The most dominant 150 protein spots were characterized by MALDI-TOF analyses. A sample of identified spots and their functional groups are specified:
1. Cytosketeletal proteins: tubulin, actin, profilin, tropomyosin. 2. Signaling proteins: enolase, rho GDP dissociation inhibitor 2, glutathione S transferase, nucleophosmin. 3. Metabolism: phosphoglycerate kinase I, glyceraldehyde-3P-dehydrogenase. 4. Protein folding: heat shock proteins (HSP 60, HSP70c), molecular chaperones (GRP78).
Conclusions: Proteomics is a highly effective method to describe the gen-expression and the cell biology of stem cells on real protein transcription. Highly purified CD34+ cells from PB demonstrate a complex proteom. Our preliminary results show that cell cycle determining chaperones (folding of other protein and responsible for their functional activation or deactivation) are dominantly expressed. Thus cytoskeletal proteins dominate the protein pattern of CB stem cells. Further Proteom-comparison with other subsets of stem cells from different origins (e.g. fetal liver, cord blood, bone marrow) are currently achieved.
Author notes
Corresponding author