Abstract
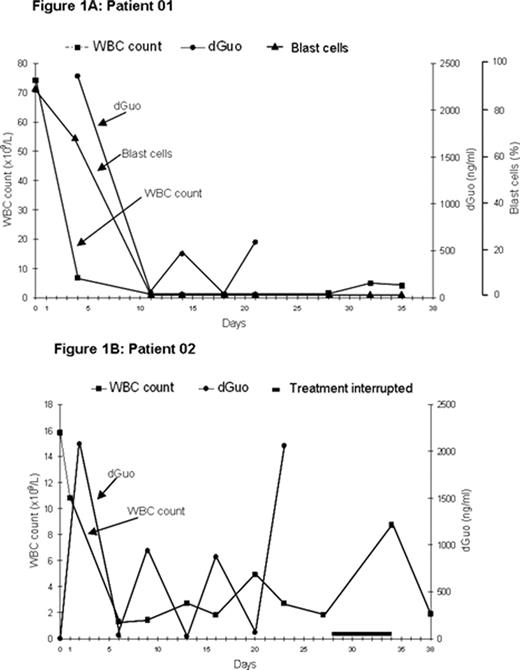
PNP represents an attractive target for drug development based on the profound, selective impairment of T-cell function seen in individuals with an inherited deficiency of PNP. Forodesine, a potent, specific transition-state analog inhibitor of PNP, is currently being investigated for safety and efficacy in T-cell and other malignancies. In a phase I/II single-center, intrapatient dose-escalation study, forodesine (starting dose: 40 mg/m2) was given to 5 patients with relapsed/refractory, aggressive T-cell leukemia/ lymphoma via a 30-min IV infusion, followed 24 hrs later by doses administered every 12 hrs for a total of 9 doses; courses could be repeated every 3–4 weeks, as required. Two patients with refractory T-cell prolymphocytic leukemia (T-PLL) and two patients with T-cell acute lymphoblastic leukemia (T-ALL) showed a decrease in leukemic or prolymphocytic counts in peripheral blood and/or bone marrow. All responders had increases in plasma 2′-deoxyguanosine (dGuo) (Cmax: 2.9 to 34 μM; baseline concentration: <.004 μM) and 4/5 responders showed elevated intracellular dGTP levels (10- to 60-fold). Forodesine was generally safe and well tolerated at all dose levels with no dose-limiting toxicities and only two adverse events (ie, acute renal failure and neutropenic infection) that were possibly drug-related. These promising results led to a phase II multi-center, open-label, repeat-dose trial in patients with advanced T-cell malignancies, in which forodesine (40 mg/m2) is infused for 30 mins for 5 days followed by at least 2 non-treatment days for up to 5 additional cycles. Out of 3 patients enrolled to-date, 2 T-ALL patients completed 6 courses of therapy with a dramatic reduction in WBC count (Fig 1A and 1B); 1 splenic T-cell lymphoma patient discontinued due to disease progression. Forodesine resulted in striking elevations in plasma dGuo. Peripheral blast cells in Patient 01 were undetectable at the start of the second course and remained undetectable after 6 courses (Fig 1A), and the bone marrow blast cells were reduced from >90% to near-normal levels. However, the contribution of a single dose of dexamethasone (40 mg IV given on Day 3) to the reduction in WBC count and blast cells is unknown. This patient’s peripheral blood hematologic profile (WBC, absolute neutrophil, platelet counts and hemoglobin level) returned to near-normal, indicating a specificity of forodesine for leukemic cell populations and supporting that forodesine is an important breakthrough in the development of less toxic ALL therapy. Additional phase II results will be presented.
Author notes
Corresponding author