Abstract
Relapse of B-lineage (CD19+) acute lymphoblastic leukemia (ALL) remains a major impediment to the therapeutic success of allogeneic umbilical cord blood transplant (UCBT). The adoptive transfer of donor-derived tumor-specific T-cells is a conceptually attractive means to improve the graft-versus-leukemia-effect at the time of minimal residual disease to improve relapse-rates without exacerbating graft-versus-host-disease. However, adoptive immunotherapy after banked UCBT has been limited by the functional naïveté of neonatal T cells and difficulty obtaining T cells from the unrelated donor. These hurdles can now be overcome by genetically rendering cord blood-derived T cells to be specific for CD19 and expanding the T cells ex vivo from small numbers of cord blood cells, in compliance with current good manufacturing practices for phase I/II trials. To generate T cells that target CD19+ malignant cells, we have used non-viral gene transfer to introduce a DNA plasmid to express a CD19-specific chimeric immunoreceptor, designated CD19R, which binds to cell-surface CD19 via an scFv, independent of MHC, and triggers T-cell activation through CD3- ζ. To safeguard the safety of recipients of adoptive immunotherapy, the DNA plasmid co-expresses the bi-functional hygromycin phosphotransferase/HSV-1 thymidine kinase (HyTK) selection/suicide gene. To assess in vivo the fate of adoptively transferred T cells in mice; a novel tri-functional gene linking firefly luciferase (ffLuc) with HyTK (ffLucHyTK) was generated. The process ex vivo to expand cord blood-derived T cells, which is currently employed at COH in human trials, uses reiterative 14-day additions of OKT3, rhIL-2, cytocidal concentrations of hygromycin, and irradiated peripheral blood mononuclear cells (PBMC) and LCL as feeder cells. The expanded genetically manipulated cord-blood derived T cells express cell-surface markers of differentiated effector cells, similar to the phenotype of CD19-specific T cells derived from PBMC. In vitro the CD19R+HyTK+ cord blood-derived T cells are activated for cytolysis and cytokine production by CD19+ tumor cells. In vivo these genetically modified T cells can be used to eradicate established CD19+ tumors and undergo ganciclovir-mediated ablation, as demonstrated by non-invasive serial imaging of luciferase-mediated bioluminescence (see Figure). These data support a clinical trial to test the safety and feasibility of adoptive transfer of CD19-specific umbilical cord-blood derived T-cells for patients with high risk B-lineage ALL undergoing UCBT.
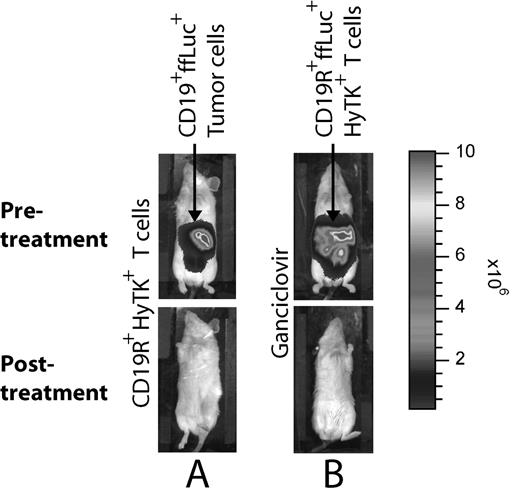
Legend: Non-invasive in vivo biophotonic imaging demonstrates that (A) CD19+ tumor expressing ffLuc gene are eliminated by CD19R+HyTK+ cord-blood derived T cells, and (B) CD19R+ffLuc+HyTK+ cord-blood derived T cells are ablated by ganciclovir. Top row: prior to adoptive immunotherapy or ganciclovir treatment. Bottom row: after adoptive immunotherapy or ganciclovir treatment. Two representative mice are shown.
Author notes
Corresponding author