Abstract
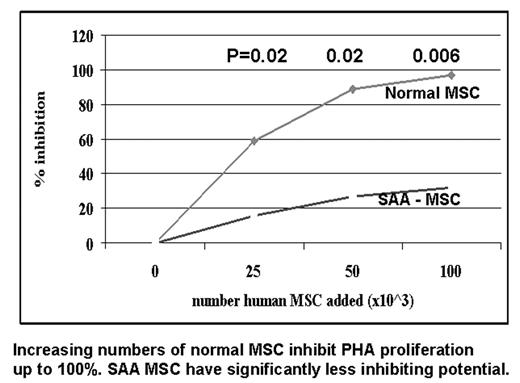
Normal bone marrow derived mesenchymal stem cells (MSC) suppress T cell function, and may provide an immunoprotected environment for hemopoietic stem cells. In this study we compare the suppressive activity on T cell function of MSC from 23 patients with severe aplastic anemia (SAA) patients, at diagnosis (n=3), following immunosuppressive therapy (IS) (n=16) or after an allogeneic bone marrow transplant (BMT) (n=4), and normal individuals. As shown in Figure 1 increasing numbers of control MSC (25, 50 and 100x10^3 cells) produced a dose dependent suppression of PHA induced T cell proliferation; when MSC were derived from SAA patients, they exhibited significantly less suppressive activity (37% vs 6%, 81% vs 24%, 96% vs 35%). The reduced ability of SAA MSC to suppress mitogen induced T cell proliferation, was seen irrespective of disease phase. Paired experiments on mixed lymphocyte reaction confirmed the inability of SAA MSC to suppress T cell function. Other abnormalities of SAA MSC included: (a) impaired capacity to down regulate CD38 expression on PHA primed T cells, (b) impaired ability to suppress gamma-IFN production in PHA coltures, which resulted in a 11 fold different gamma-IFN concentration in the cultures; (c) no effect on T cell mediated inhibition of hemopietic colony formation. Finally SAA MSC produced significantly (1 log) less adenosin diphosphate -ribosyl cyclase (cADPR), a promoter of in vitro hematopoiesis. MSC mediated suppression of PHA induced T cell proliferation, was restored to control levels in 3 of 4 patients post-BMT. In conclusion, the ability of MSC to down regulate T cell priming, proliferation and cytokine release is deficient in patients with SAA, persists indefinitively after immunosuppressive therapy, but may be restored after BMT. Whether this defect plays a role in the pathogenesis of marrow failure, remains to be determined.
This work was supported by Fondazione CARIGE Genova.
Author notes
Corresponding author