Abstract
Patients with a clinical diagnosis of TTP presenting to The Ohio State University were enrolled and treated on a prospective trial of concurrent plasma exchange (PE) and adjuvant immunosuppression.
Patients: Between April 2003 and August 2004, 15 patients were entered and followed on this study. The median age was 46 years (range, 19–59), 11/15 were female, 7/15 were African American, and 9/15 were obese (body mass index >30 kg/m2). Nine patients had been previously treated for TTP.
Method: All patients with TTP were treated initially with concurrent PE and corticosteroids. Daily exchanges of one plasma volume using cryosupernatant plasma were performed with concurrent prednisone at a dose of 1 mg/kg/day. PE was continued until a clinical remission was achieved as defined by: 1) normal platelet count 2) normalization of LDH for 2 consecutive determinations, and 3) improvement or stabilization of renal or neurologic abnormalities. After criteria for remission were achieved patients had 2 additional procedures performed every other day and the prednisone tapered over the next 4 weeks. Patients with exacerbations (defined as the need to reinstate PE within 30 days discontinuation) of TTP had prednisone discontinued and began oral cyclosporine (CSA) at a dose of 2–3 mg/kg/day orally concurrent with the resumption of PE. After achieving a second remission, PE was tapered as described previously and CSA was continued for a total of 6 months of therapy. Serial measurements of ADAMTS13 activity and inhibitor assays were obtained prior to the initiation of PE and at regular intervals during follow-up.
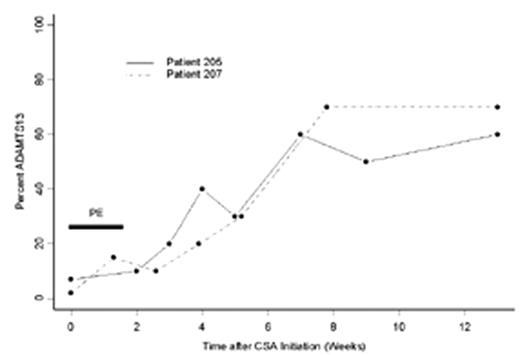
Results: ADAMTS13 data is available for the first 6 patients treated with PE + prednisone. A summary of clinical outcomes and ADAMTS13 activity may be found in Table 1. Of the patients suffering exacerbations after initial therapy with PE + prednisone, ADAMTS13 data is available from the first 2 of 5 patients treated with PE + CSA and is shown in Figure 1.
Clinical Responses and ADAMTS13 Activity
| . | Inhibitor . | Remission . | Exac. . | Relapse . | Med. ADAMTS13-Pre . | Med. ADAMTS13-Remission . | Med. Remission (mo) . |
|---|---|---|---|---|---|---|---|
| * samples tested to date **median ADAMS13 | |||||||
| PE+Pred. | 5/6* | 14/15 | 7/15 | 2/15 | 5% (range, 2-7%)** | 5% (range, 2-7%)** | 1(range, 1-9 ) |
| PE+CSA | 2/2* | 5/5 | 0/5 | 0/5 | Figure 1 | Figure 1 | 6 (range, 3-10) |
| . | Inhibitor . | Remission . | Exac. . | Relapse . | Med. ADAMTS13-Pre . | Med. ADAMTS13-Remission . | Med. Remission (mo) . |
|---|---|---|---|---|---|---|---|
| * samples tested to date **median ADAMS13 | |||||||
| PE+Pred. | 5/6* | 14/15 | 7/15 | 2/15 | 5% (range, 2-7%)** | 5% (range, 2-7%)** | 1(range, 1-9 ) |
| PE+CSA | 2/2* | 5/5 | 0/5 | 0/5 | Figure 1 | Figure 1 | 6 (range, 3-10) |
Conclusions: In this prospective study, no patients had significant improvement in ADAMTS13 activity despite achieving remission with PE and corticosteroids. These data showing persistently low ADAMTS13 activity at the time of remission may have contributed to the high rate of exacerbations in this group of patients. In the analysis of the first 2 patients treated with concurrent PE and CSA, there was clear and sustained improvement in ADAMTS13 activity that began after the discontinuation of PE and correlated with the clinical remission of TTP.
Author notes
Corresponding author