Abstract
Introduction: Though response may occur with standard therapy, early relapse is common with advanced stage MCL, suggesting the importance of drug resistance in this disease process.It appears that repeated cycles of aggressive chemotherapy to a point of maximum response, followed by HDT and AHSCT, provides improved disease free intervals, though the impact on overall survival (OS) remains uncertain. A concern with this approach is that the eventual high relapse rate seen in most trials may be due to the emergence of drug resistance prior to HDT. In an attempt to circumvent the problem of drug resistance due to multiple cycles of chemotherapy, we designed a dose dense approach using only one cycle of an aggressive induction regimen. Patients demonstrating at least PR and a BM uninvolved by morphology and flow cytometry studies proceeded with chemotherapy-primed PBPC collection, before receiving HDT and AHSCT.
Patients and Methods: HIDAC 3 gm/m2 over 1 hour q12 hours for 12 doses in combination with mitoxantrone 12mg/m2 daily for 3 days were given as induction therapy. Responders were mobilised with either VP-16 2 gm/m2 or cyclophosphamide 4 gm/m2 followed by G-CSF 10 mcg/kg daily until stem cell collection. The preparative regimen consisted of BCNU 15mg/kg over 2 hours D-6, VP-16 60mg/kg over 4 hours D-4 and cyclophosphasmide 100mg/kg over 2 hours D-2.
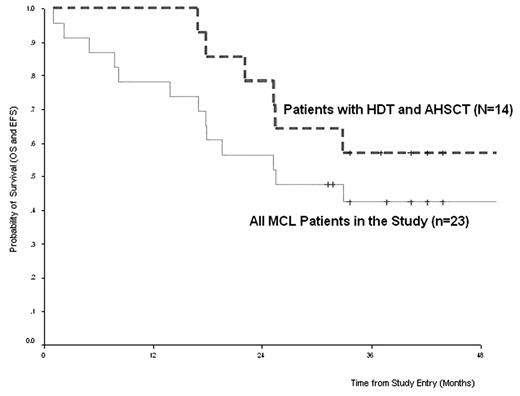
Results: Twenty one stage IV patients and 2 stage III patients were enrolled, including 7 with relapsed/refratory disease. Median age was 56 yo(40–74). Nine (39%) patients achieved CR and 11 (48%) patients achieved PR with all showing >80% reduction in tumor size. Three patients died after induction: 1 from sepsis; 2 from disease progression. Seventeen (74%) of the 20 patients with CR or > 50% PR proceeded to PBPC mobilisation whereas 3 were deemed too ill to undergo HDT (2 of these were in CR from induction). A total of 14 (10 previously untreated and 4 had failed prior therapy) eventually had adequate stem cell collected and underwent planned HDT and were fully evaluable for outcome. There was no TRM to HDT and while only 6 patients entered HDT in CR, all 14 patients attained a CR at recovery from transplant. With a median follow-up from study entry for these 14 autotransplant patients of 36 months (17–68), 8 patients are still alive and in CR. The estimated 4 year OS and event free survival (EFS) for these 14 patients were both 64% and the median survival was 57 months.(see figure)
Conclusions: The study shows that a dose dense, high intensity approach for advanced MCL provides a very high CR and PR rate. Those who were able to complete this protocol have a high chance of achieving favorable disease free survival.
Figure.
Author notes
Corresponding author