Human T-cell alloreactivity plays an important role in many disease processes, including the rejection of solid organ grafts and graft-versus-host disease (GVHD) following allogeneic stem cell transplantation. To develop a better understanding of the T cells involved in alloreactivity in humans, we developed a cytokine flow cytometry (CFC) assay that enabled us to characterize the phenotypic and functional characteristic of T cells responding to allogeneic stimuli. Using this approach, we determined that most T-cell alloreactivity resided within the CD4+ T-cell subset, as assessed by activation marker expression and the production of effector cytokines (eg, tumor necrosis factor α [TNF]α) implicated in human GVHD. Following prolonged stimulation in vitro using either allogeneic stimulator cells or viral antigens, we found that coexpression of activation markers within the CD4+ T-cell subset occurred exclusively within a subpopulation of T cells that significantly increased their surface expression of CD4. We then developed a simple sorting strategy that exploited these phenotypic characteristics to specifically deplete alloreactive T cells while retaining broad specificity for other stimuli, including viral antigens and third-party alloantigens. This approach also was applied to specifically enrich or deplete human virus-specific T cells.
Introduction
The recognition of alloantigens by human T cells forms the basis for several clinically significant disease processes, including graft-versus-host disease (GVHD) arising in the setting of stem cell transplantation (SCT).1,2 In SCT, numerous interventions have been attempted to reduce the risk of GVHD, most of which have targeted the number and/or function of T cells transferred from the donor to the recipient.3 Inhibitors of T-cell activation, including cyclosporine A or tacrolimus, are administered to nearly all SCT recipients (reviewed in Champlin et al4 and Ho and Soiffer5 ). Additional strategies have involved the elimination of T cells within the transferred graft, either by negative depletion of CD3+ T cells or by the transfer of positively selected CD34+ stem cells.6-8 In other cases, polyclonal or monoclonal antibodies used to purge the allograft or administered to the recipient shortly after graft infusion, including antithymocyte globulins9 or Campath-1H, 10 effectively result in the depletion of graft-derived T cells. While these strategies reduce the risk of GVHD after SCT, 5,11 the benefits of decreases in morbidity due to GVHD are often offset by the simultaneous nonspecific reduction in the numbers of nonalloreactive T cells, leading to an increased risk of infection and relapse following T-cell depletion.12,13 For many SCT candidates, suitable matched donors are not available, precluding the application of this potentially curative treatment modality. Consequently, most transplantations are performed using matched sibling or unrelated donors due to our inability to selectively reduce alloreactivity in the setting of mismatched transplantation. Thus, practical methods that allow us to specifically deplete alloreactive T cells, while sparing other T-cell populations, could have significant clinical use in the setting of SCT. Here, we demonstrate that alloreactive CD4+ T cells may be identified by CD4 up-regulation and coexpression of surface activation markers. We also show that sort-based depletion of cells with this phenotype leaves behind a CD4+ and CD8+ T-cell population that is diverse and capable of responding to pathogens but that is functionally anergic following restimulation with alloantigens. This approach also was shown to be similarly useful for the depletion and enrichment of virus-specific human T cells.
Materials and methods
Preparation of responder and stimulator cells
This research was approved by the institutional review board at the M.D. Anderson Cancer Center. Informed consent was obtained according to principles outlined in the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque technique sedimentation from heparinized whole blood of healthy volunteer cytomegalovirus (CMV)–seropositive donors. For stimulator cells, PBMCs from 10 unique donors were irradiated at 25 Gy, pooled, and cryopreserved for allogeneic stimulation experiments. For control stimulations, autologous PBMCs obtained from healthy donors were similarly irradiated and cryopreserved.
Cytokine flow cytometry assay
For initial experiments establishing the kinetics of cytokine production and activation marker coexpression in the cytokine flow cytometry assay, 106 freshly isolated responder PBMCs were co-incubated with either CMV lysates (BioWhittaker, Walkersville, MD) or an equal number of thawed autologous or pooled allogeneic PBMCs in 24-well tissue culture plates in 2 mL of media (RPMI1640; GIBCO Life Technologies, Grand Island, NY) supplemented with 10% human AB serum, l-glutamine, penicillin, and streptomycin (Sigma, St Louis, MO). Stimulator cells were labeled with the membrane dye PKH-2 (Sigma) to enable identification of activation within responder cells. Following stimulation, cells were washed and brefeldin A (Sigma) was added to enable accumulation of effector cytokines in the cytoplasm. We then permeabilized cells with FACSPerm Solution II (BD Biosciences, San Jose, CA) and examined responder T cells for the simultaneous expression of surface activation markers and intracellular cytokine expression.
Assessment of T-cell activation by flow cytometry
FACS analyses were performed using fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, peridinin chlorophyll protein (PerCP)–, and antigen-presenting cell (APC)–conjugated monoclonal antibodies (MAbs) specific for human CD4, CD8, CD14, CD38, CD25, CD69, HLA-DR, CD71, CD58, CD122, CD152, CD103, CD134, anti-interferon [IFN]γ, anti–tumor necrosis factor [TNF]α, and anti–interleukin 2 (IL-2) (BD Biosciences). After staining, cells were washed, resuspended in phosphate-buffered saline (PBS) with 1% paraformaldehyde, and analyzed by 4-color flow cytometry on a FACSCalibur cytometer using Cell Quest software (both BD Biosciences) and FlowJo software (Treestar, San Carlos, CA). For most analyses, at least 100 000 total events were analyzed, with sequential gating of PBMCs in a lymphocyte region (by scatter), on CD14– events to exclude monocytes and on antigen-specific T cells (by assessing CD4+ or CD8+ T cells staining positive for intracellular TNFα, IL-2, or IFNγ). We assessed nonspecific activation by incubation of paired samples that were either unstimulated or stimulated with autologous control cells. The observed frequency of cytokine-producing T cells following autologous and CMV control stimulation was generally fewer than 0.2%.
CFSE proliferation assay
To assess proliferation in responder cells, we labeled them with carboxyfluorescein diacetate (CFSE) (Molecular Probes, Eugene, OR), a highly fluorescent dye that is transferred to daughter cells, resulting in a linear decrease in fluorescence. We labeled PBMCs with 0.6 μM CFSE for 10 minutes at room temperature, quenched the reaction with AB serum, and washed them twice with RPMI 1640 containing 10% AB serum. The starting frequency of alloreactive T cells was estimated based on the median of fluorescence intensity (MFI) and the percentage of proliferating cells after 7 days of mixed lymphocyte reaction (MLR) culture. The precursor frequency of the starting population was calculated based on the following formula, where a = MFI (CD4hi population), b = MFI (CD4int population), c = frequency of CD4hiCD38+ T cells following alloantigen stimulation (in percent), and n = cell divisions in the alloreactive T-cell population (log2[a/b]):
Specific depletion of alloreactive or CMV-specific CD4+ T cells
To deplete antigen-specific CD4+ T cells, we stimulated 108 responder PBMCs with CMV lysates, an equal number of PBMCs from an allogeneic pool, or autologous PBMCs. After 7 days, we harvested and washed responder cells and labeled them with anti-CD4PerCP and CD38APC MAb (BD Biosciences). We then sorted cells using a FACSAria flow cytometer (BD Biosciences), excluding CD4hiCD38+ cells. Prior to sort-based allodepletion, cells were resuspended in PBS containing 0.1% AB serum and disodium edetate (5 mM final concentration) (Sigma). Following sorting, cells were washed twice in PBS prior to further functional assessment. For spectratyping studies, we selectively purified both CD4hiCD38+ and CD4intCD38– populations. For assessment of residual antigen-specific T-cell function, we rested the residual population of cells for 16 hours and then restimulated them with either thawed allogeneic or autologous PBMCs or CMV lysates. Secondary stimulations were performed for either 3 or 7 days and assessed as described for primary stimulations using the cytokine flow cytometry (CFC) assay.
Preclinical assessment of alloreactive T-cell depletion
We obtained cells from a healthy donor (donor C) and stimulated them with thawed and irradiated PBMCs from clinical apheresis products obtained from 2 different HLA-mismatched donors (A and B). Following 7 days of stimulation, we depleted CD4highCD38+ alloreactive T cells by sorting and performed secondary stimulations and assessed residual autologous, CMV-specific, and third-party alloreactivity by CFC.
Spectratyping assay
Immediately after sorting, we extracted total RNA using a commercial kit (Tel-Test, Friendswood, TX) and prepared cDNA using reverse transcription (Applied Biosystems, Foster City, CA). We then amplified the CDR3 regions in 23 T-cell receptor (TCR) Vβ subsets by polymerase chain reaction (PCR) and subjected the resulting PCR products to capillary electrophoresis and quantitative densitometry to assess the fragment length diversity within each of the TCR Vβ families as previously described.14,15
Statistical analyses
Data were collected, analyzed, and displayed using Prism software (GraphPad, San Diego, CA) and Illustrator software (Adobe, Seattle, WA) using Macintosh computers (Apple, Cupertino, CA). Nonparametric comparisons were performed using the Mann-Whitney U test. We performed intergroup comparisons using a nonpaired, 2-tailed one-way analysis of variance (ANOVA) test. Values of P less than .05 were considered significant.
Results
Development of a cytokine flow cytometry assay of human T-cell alloreactivity
CFC was first developed by Waldrop et al16 to quantitate human virus-specific T cells. In subsequent clinical studies, we demonstrated that risk for opportunistic infection was associated with diminished CMV-specific T-cell responses assessed by CFC17 and that CFC accurately reflected clinically meaningful immune reconstitution. While CFC methods now have been applied to study a wide range of human pathogens, CFC methods have not been routinely applied to study alloreactive T-cell responses in humans. To better characterize human T-cell alloreactivity, we first isolated PBMCs from a group of 10 HLA-disparate individuals, irradiated them, and pooled them to provide a stimulus for responder T cells. We isolated responder PBMCs from healthy donors and cocultured them with the pooled allogeneic stimulator cells for varying periods. Following stimulation, we examined responder T cells for the simultaneous expression of phenotypic markers associated with T-cell maturation or activation and the production of effector cytokines by CFC.
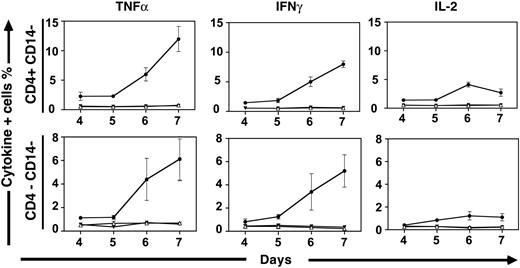
Kinetics of human primary and secondary alloreactivity may be assessed using cytokine flow cytometry. We initially labeled stimulator cells with PKH-2 to determine how long irradiated allogeneic stimulators persisted in MLRs. PKH-2+ stimulator cells were present in declining numbers over the first 24 to 48 hours of stimulation but were undetectable thereafter (data not shown). These data suggest that indirect allorecognition is the primary mechanism operative in MLR, following an initial period where both direct and indirect allorecognition may occur. Following stimulation with pooled allogeneic stimulator cells, the production of effector cytokines, including TNFα, was induced in responder cells (6.85% of CD4+ T cells; data not shown). In contrast, we observed no significant TNFα production in CD4+ T cells stimulated with autologous cells, regardless of whether autologous stimulators were irradiated or not (≤ 0.32% of CD4+ T cells; data not shown). Consistent with prior observations derived from lymphocyte proliferation assays, cytokine production was evident following approximately 5 days of allogeneic stimulation. The kinetics of effector cytokine production were similar, irrespective of whether we examined IL-2, IFNγ, or TNFα production (Figure 1). Cytokine production was seen exclusively within T cells, as assessed by forward and side scatter and by the lack of CD14 expression. Most cytokine production was seen in CD4+CD14– T cells, although a smaller proportion of CD8+ T cells (demarcated by the CD4–CD14– population within the T-cell gate) also produced effector cytokines. Interestingly, we found that the kinetics of the secondary alloresponse were accelerated, regardless of whether cells were initially stimulated with either allogeneic stimulator cells or viral antigens (data not shown). While little cytokine production was evident prior to 5 days in the primary allogeneic response, cells stimulated previously with either CMV or allogeneic responders were observed to produce cytokines within 3 days following secondary stimulation. To characterize residual alloreactivity in subsequent experiments examining depletion strategies, secondary responses were assessed at both 3 days and 7 days, with similar results obtained at both intervals.
Kinetics of cytokine production in alloreactive human T cells. Cytokine production within donor CD4+CD14– and CD4–CD14– cells are shown at various intervals following stimulation with pooled allogeneic PBMCs. The frequencies of T cells producing intracellular TNFα, IFNγ, and IL-2 were assessed by CFC. In each graph, frequencies of cells responding to stimulation with allogeneic pooled PBMC (•) and autologous PBMCs that were either irradiated (▾) or not irradiated (▵) are shown. Medians and standard deviations shown were derived from 6 experiments performed using unique donors and the same allogeneic PBMC pool.
Kinetics of cytokine production in alloreactive human T cells. Cytokine production within donor CD4+CD14– and CD4–CD14– cells are shown at various intervals following stimulation with pooled allogeneic PBMCs. The frequencies of T cells producing intracellular TNFα, IFNγ, and IL-2 were assessed by CFC. In each graph, frequencies of cells responding to stimulation with allogeneic pooled PBMC (•) and autologous PBMCs that were either irradiated (▾) or not irradiated (▵) are shown. Medians and standard deviations shown were derived from 6 experiments performed using unique donors and the same allogeneic PBMC pool.
Activation marker expression and proliferation occur exclusively in CD4hi T cells following chronic stimulation
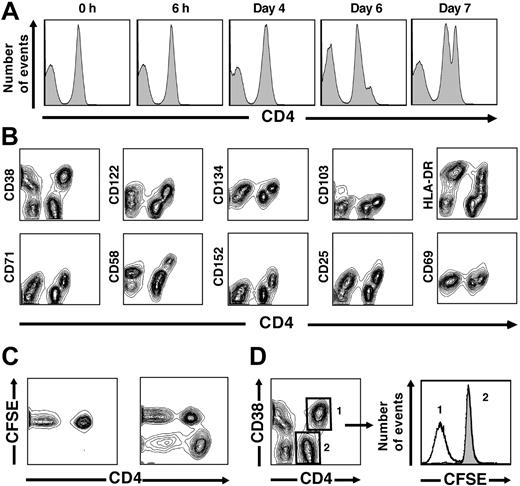
Following short-term stimulation, such as the 6-hour period used to stimulate CMV-specific CD4+ T cells with viral antigens in CFC assays, the intensity of CD4 expression within CD4+ lymphocytes maintains a fairly narrow and Gaussian distribution.17 In contrast, following chronic stimulation with either CMV antigens or pooled allogeneic stimulator cells, we noted that the peak of fluorescence intensity demarcating CD4 expression initially widened and then became segregated into 2 distinct populations with intermediate (CD4int) and high (CD4hi) staining intensity (Figure 2A). At 4 days, CD4 intensity remained Gaussian, similar to earlier intervals. By 6 days, a clear shoulder consisting of the CD4hi population was evident, and this population was well defined by 7 days (Figure 2A). We excluded the possibility that this increase in surface intensity was simply related to an increased size of a CD4+ blast population, as the increase in fluorescence intensity (up to 10-fold) was disproportionate to cell size as assessed by scatter and not seen in similar analyses of other surface markers that would also be expected to increase in proportion to blast size (data not shown).
CD4 up-regulation occurs following chronic stimulation in vitro. (A) Histograms represent CD4 fluorescence intensity following various stimulation periods. A distinct CD4hi population starts to become evident at day 6 and is clearly evident at day 7 in data representative of experiments from 6 unique donors. (B) Up-regulation of activation markers occurs uniquely on CD4hi T cells. Following 7 days of stimulation with an allogeneic PBMC pool, up-regulation of 10 activation markers (y-axes) was assessed with respect to CD4 fluorescence intensity (x-axis). In each case, activation-marker coexpression was restricted within the CD4+ T-cell subset to the CD4hi population. Data from 1 donor shown are representative of experiments from 8 unique donors stimulated in the same fashion. (C) Proliferation of alloreactive T cells is restricted to the CD4hi subset. A decrease in fluorescence intensity of CFSE, a dye used to label responder cells, is seen within the CD4+ T-cell population only in the CD4hi subset following 7 days of stimulation. (D) Within the CD4+ T-cell population, CFSElow cells are confined to the alloreactive CD4hiCD38+ T-cell subset following allogeneic stimulation. The median number of divisions, calculated by the change in CFSE fluorescence intensity in the 2 peaks illustrated at right, is 4.1 ± 0.5, based on experiments from 5 unique donors. Assuming that nondividing cells did not die during the stimulation period, we estimate that the original precursor frequency of alloreactive cells was 6.9% ± 1.5% of the original CD4+ T-cell population.
CD4 up-regulation occurs following chronic stimulation in vitro. (A) Histograms represent CD4 fluorescence intensity following various stimulation periods. A distinct CD4hi population starts to become evident at day 6 and is clearly evident at day 7 in data representative of experiments from 6 unique donors. (B) Up-regulation of activation markers occurs uniquely on CD4hi T cells. Following 7 days of stimulation with an allogeneic PBMC pool, up-regulation of 10 activation markers (y-axes) was assessed with respect to CD4 fluorescence intensity (x-axis). In each case, activation-marker coexpression was restricted within the CD4+ T-cell subset to the CD4hi population. Data from 1 donor shown are representative of experiments from 8 unique donors stimulated in the same fashion. (C) Proliferation of alloreactive T cells is restricted to the CD4hi subset. A decrease in fluorescence intensity of CFSE, a dye used to label responder cells, is seen within the CD4+ T-cell population only in the CD4hi subset following 7 days of stimulation. (D) Within the CD4+ T-cell population, CFSElow cells are confined to the alloreactive CD4hiCD38+ T-cell subset following allogeneic stimulation. The median number of divisions, calculated by the change in CFSE fluorescence intensity in the 2 peaks illustrated at right, is 4.1 ± 0.5, based on experiments from 5 unique donors. Assuming that nondividing cells did not die during the stimulation period, we estimate that the original precursor frequency of alloreactive cells was 6.9% ± 1.5% of the original CD4+ T-cell population.
We then stimulated PBMCs from healthy donors with pools of allogeneic stimulator cells for 7 days and simultaneously assessed the intensity of CD4 expression and the expression of 10 different T-cell activation markers (Figure 2B). As expected, given known variances in the kinetics of activation marker up-regulation, some markers were expressed more intensely following chronic activation using either viral antigens or alloantigens (eg, CD38, CD58, and HLA-DR), while others (eg, CD69 and CD103) were present at only minimally increased intensity on the surface of activated T cells. Regardless of the intensity of activation marker expression following allogeneic stimulation, we observed increased expression of each activation marker only on the subset of CD4+ T cells that up-regulated surface CD4 following stimulation (ie, CD4hi T cells) (Figure 2B). We consistently observed a close association between CD4 up-regulation and activation marker expression; for each marker assessed, CD4hi cells all were activation-marker positive, while CD4int cells were activation-marker negative. We observed similar results when PBMCs from CMV-seropositive donors were stimulated for 7 days with CMV lysates (data not shown).
We additionally examined the association between CD4 up-regulation, secondary activation-marker expression, and effector cytokine production. In each case, cytokine production within the CD4hi population was contained within the subpopulation of cells coexpressing activation markers, further demonstrating the close association between CD4 up-regulation, activation-marker coexpression, and effector cytokine production (data not shown). Similar associations were seen in more limited analyses using IFNγ and IL-2 production as an end point.
These experiments suggested that up-regulation of CD4 was a uniform and reliable indicator of viral and alloantigen activation in CD4+ T cells. To determine whether CD4 up-regulation also was associated with the proliferation of activated CD4+ T cells, we labeled healthy donor PBMCs using the fluorescent dye CFSE prior to stimulation with either CMV or pools of alloantigenic PBMCs. We found decreases in CFSE fluorescence intensity only in CD4hi cells, suggesting that proliferation, similar to activation-marker expression, was restricted exclusively to the CD4hi subset (Figure 2C). Conversely, we did not observe CFSElow cells within the CD4int population, suggesting that these cells had not proliferated, consistent with the lack of activation-marker expression on these cells. Because CFSE fluorescence intensity declines linearly following cell division, we were able to calculate the number of CD4hi cell divisions during the stimulation period.18 Based on 5 experiments from unique donors, we determined that cells divided approximately 4 times during 7 days (Figure 2D). Assuming that nondividing cells did not preferentially die during the culture period, we extrapolated the starting frequency of alloantigen-specific T cells from the final frequencies of CD4hiCD38+ T cells within the total CD4+ population. We estimate the median frequency of alloantigen-specific CD4+ T cells at 6.9% ± 1.5% (data not shown). This estimate is consistent with theoretical estimates of the frequency of alloantigen-specific cells in the TCR repertoire but significantly higher than prior estimates of alloreactive T-cell frequencies obtained using limiting dilution assay methods.19
Elimination of specific CD4+ and CD8+ T-cell function by depletion of activated CD4hi T cells
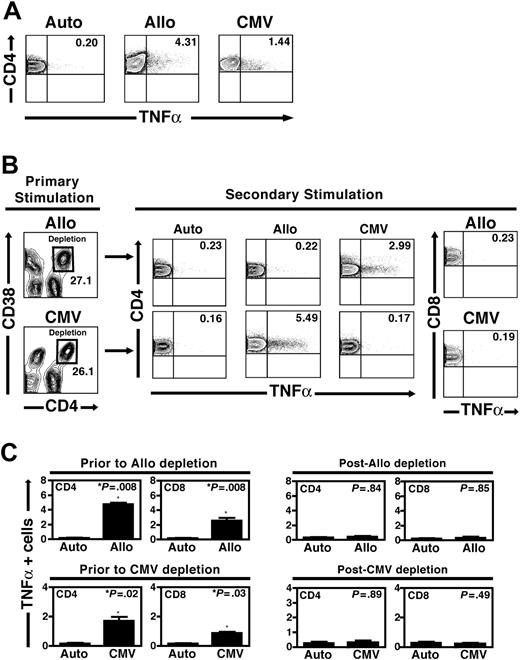
Because CD4 up-regulation appeared to be a hallmark of activation following either viral or alloantigen stimulation, we reasoned that depleting the CD4hi population following activation would selectively eliminate T cells responding to a specific stimulus. To test this strategy, we obtained PBMCs from CMV-seropositive healthy donors and stimulated them with either CMV lysates or irradiated pools of allogeneic stimulator cells. Instead of using CD4 expression independently, we sorted cells on the basis of the simultaneous expression of a secondary activation marker to define a sort gate that most clearly resolved subpopulations of activated CD4+ T cells. Based upon our analyses of multiple activation markers and CD4 up-regulation (Figure 2B), we found that CD38 coexpression was particularly well suited for this purpose. Following 7 days of stimulation with a pool of allogeneic stimulator cells, we stained the PBMC population with antibodies to surface CD4 and CD38 and then used high-speed sorting to deplete cells with the CD4hiCD38+ phenotype. A typical experiment is shown (Figure 3). Few PBMCs from a healthy CMV-seropositive donor produced intracellular TNFα following autologous stimulation (0.2%), while significant frequencies of CD4+ T cells responded to stimulation with pooled allogeneic stimulators or CMV lysates (4.31% and 1.44% of CD4+ T cells, respectively; Figure 3A). Following 7 days of stimulation with either pooled allogeneic PBMCs or CMV lysates, we depleted CD4hiCD38+ T cells by high-speed sorting (Figure 3B). Following depletion, we again stimulated the residual PBMCs with either the primary stimulus or a different secondary stimulus. We assessed secondary responses to CMV or alloantigen challenge using CFC assays examining the production of TNFα within the CD4+ T-cell population.
Specific depletion of alloreactive and/or CMV-specific CD4+ T cells. (A) The baseline frequencies of responder CD4+ T cells from a healthy CMV-seropositive donor responding to 7 days of stimulation with autologous PBMCs, pooled allogeneic PBMCs, and CMV antigens are shown. In each case, the TNFα+ frequency within the CD4+ T-cell population is shown (upper right quadrants). (B) Specific depletion of CD4hiCD38+ T cells following allogeneic or CMV stimulation. Following primary stimulation with either pooled allogeneic PBMCs (top) or CMV lysates (bottom), high-speed sorting was used to deplete the cells contained within the CD4hiCD38+ population (black squares). Residual cells, including CD4intCD38– cells and all CD8+ T cells, were then restimulated with autologous PBMCs, pooled allogeneic PBMCs, and CMV antigens. Following primary allogeneic stimulation and depletion of CD4hiCD38+ cells, secondary stimulation induced a CMV-specific CD4+ T-cell response slightly higher than that at baseline (2.99% versus 1.44%), while secondary stimulation with the allogeneic PBMC pool was reduced to a level similar to that following control autologous stimulation (0.22% versus 0.23%). Conversely, depletion of CD4hiCD38+ cells following primary CMV stimulation resulted in preserved secondary CD4+ T-cell responses to allogeneic stimulation (5.49%) but not CMV stimulation (0.17% versus 0.16% following autologous stimulation). As shown in the panels on the right, depletion of the CD4hiCD38+ T-cell subset following either allogeneic or CMV stimulation also resulted in the loss of a detectable CD8+ T-cell response following secondary restimulation with the same antigen (0.23% for allogeneic and 0.19% after CMV stimulation), similar to that in control samples (data not shown). Data shown are representative of 5 depletion experiments following allogeneic stimulation and 4 experiments depleting CMV-specific T cells from unique donors. (C) Aggregate results from 9 separate experiments are shown (5 from unique donors depleting allogeneic CD4hiCD38+ responders, 4 from depleting CMV-specific CD4hiCD38+ responders). Displayed are the medians and standard deviations of TNFα-producing cells within CD4+ and CD8+ T-cell subsets prior to either allogeneic depletion (top left panels) or CMV depletion (bottom left panels). Following depletion of either allogeneic or CMV-specific CD4hiCD38+ T cells, responses to the primary stimulus in both CD4+ and CD8+ T-cell compartments during secondary stimulation were reduced to a level indistinguishable from that seen following control autologous stimulation (right panels).
Specific depletion of alloreactive and/or CMV-specific CD4+ T cells. (A) The baseline frequencies of responder CD4+ T cells from a healthy CMV-seropositive donor responding to 7 days of stimulation with autologous PBMCs, pooled allogeneic PBMCs, and CMV antigens are shown. In each case, the TNFα+ frequency within the CD4+ T-cell population is shown (upper right quadrants). (B) Specific depletion of CD4hiCD38+ T cells following allogeneic or CMV stimulation. Following primary stimulation with either pooled allogeneic PBMCs (top) or CMV lysates (bottom), high-speed sorting was used to deplete the cells contained within the CD4hiCD38+ population (black squares). Residual cells, including CD4intCD38– cells and all CD8+ T cells, were then restimulated with autologous PBMCs, pooled allogeneic PBMCs, and CMV antigens. Following primary allogeneic stimulation and depletion of CD4hiCD38+ cells, secondary stimulation induced a CMV-specific CD4+ T-cell response slightly higher than that at baseline (2.99% versus 1.44%), while secondary stimulation with the allogeneic PBMC pool was reduced to a level similar to that following control autologous stimulation (0.22% versus 0.23%). Conversely, depletion of CD4hiCD38+ cells following primary CMV stimulation resulted in preserved secondary CD4+ T-cell responses to allogeneic stimulation (5.49%) but not CMV stimulation (0.17% versus 0.16% following autologous stimulation). As shown in the panels on the right, depletion of the CD4hiCD38+ T-cell subset following either allogeneic or CMV stimulation also resulted in the loss of a detectable CD8+ T-cell response following secondary restimulation with the same antigen (0.23% for allogeneic and 0.19% after CMV stimulation), similar to that in control samples (data not shown). Data shown are representative of 5 depletion experiments following allogeneic stimulation and 4 experiments depleting CMV-specific T cells from unique donors. (C) Aggregate results from 9 separate experiments are shown (5 from unique donors depleting allogeneic CD4hiCD38+ responders, 4 from depleting CMV-specific CD4hiCD38+ responders). Displayed are the medians and standard deviations of TNFα-producing cells within CD4+ and CD8+ T-cell subsets prior to either allogeneic depletion (top left panels) or CMV depletion (bottom left panels). Following depletion of either allogeneic or CMV-specific CD4hiCD38+ T cells, responses to the primary stimulus in both CD4+ and CD8+ T-cell compartments during secondary stimulation were reduced to a level indistinguishable from that seen following control autologous stimulation (right panels).
Following alloantigen stimulation and the depletion of CD4hiCD38+ T cells, the residual population failed to respond to repeat alloantigen challenge above the baseline level of cytokine production seen with autologous stimulation (0.22% of CD4+ T cells versus 0.23% for autologous restimulation; Figure 4B). Importantly, CMV reactivity within the depleted CD4+ T-cell population was preserved and was slightly higher than the baseline frequency (2.99% of CD4+ T cells), as expected, given the depletion of a CD4+ subpopulation devoid in CMV specificity (Figure 3B). We also performed the same experiment in the reverse direction, stimulating first with a CMV lysate and then restimulating the population depleted of CD4hiCD38+ T cells with autologous stimulators, the allogeneic PBMC pool, or CMV antigens. We found that CMV reactivity was eliminated following depletion (0.17% TNFα+ T cells upon restimulation versus 0.16% for the autologous control sample) but that responses to alloantigens were preserved (5.49% of CD4+ T cells producing TNFα upon restimulation; Figure 3B), demonstrating that this approach may be used to deplete cells of any specificity.
TCR spectratyping reveals the presence of a diverse TCR repertoire following depletion of alloreactive or CMV-specific T cells. (A) Molecular diversity of CD4hiCD38+ T cells. Purified CD4hiCD38+ T cells obtained following CMV stimulation demonstrated more skewing from a diverse Gaussian pattern relative to the CD4hiCD38+ T-cell subset obtained after allogeneic stimulation. (B) Diverse repertoire of residual CD4intCD38– T cells following depletion of reactive CD4hiCD38+ T cells. The TCR repertoire of CD4intCD38– T cells, obtained after either CMV or allogeneic stimulation, was extremely diverse, as evidenced by a normal Gaussian TCR spectratype. In each example, representative TCR Vβ subsets from 12 of 23 assessed TCR Vβ subsets. Results are from a single donor and representative of 2 similar experiments.
TCR spectratyping reveals the presence of a diverse TCR repertoire following depletion of alloreactive or CMV-specific T cells. (A) Molecular diversity of CD4hiCD38+ T cells. Purified CD4hiCD38+ T cells obtained following CMV stimulation demonstrated more skewing from a diverse Gaussian pattern relative to the CD4hiCD38+ T-cell subset obtained after allogeneic stimulation. (B) Diverse repertoire of residual CD4intCD38– T cells following depletion of reactive CD4hiCD38+ T cells. The TCR repertoire of CD4intCD38– T cells, obtained after either CMV or allogeneic stimulation, was extremely diverse, as evidenced by a normal Gaussian TCR spectratype. In each example, representative TCR Vβ subsets from 12 of 23 assessed TCR Vβ subsets. Results are from a single donor and representative of 2 similar experiments.
Our initial experiments demonstrated that the bulk of cytokine production among alloantigen-stimulated PBMCs occurred in CD4+ and not in CD8+ T cells. However, significant numbers of CD8+ T cells produced IL-2, IFNγ, and TNFα following allogeneic stimulation of unmanipulated responder cells (Figure 1). Given the established role of CD4+ T cells in maintaining the number and function of CD8+ T cells, we examined the impact of CD4 allodepletion alone on the function of residual, nondepleted CD8+ T cells. Following the depletion of alloantigen-specific CD4+ T cells, we found that CD8+ T cells within the residual PBMC population were incapable of activation, as assessed by intracellular TNFα expression following allogeneic stimulation approximating that following autologous restimulation, despite the fact that we observed up-regulation of activation markers including CD38 within these CD8+ T cells during primary exposure to pooled alloantigenic stimulators (0.23% TNFα+ T cells in the CD8+ population; Figure 4B). Importantly, we found that these CD8+ T cells could still respond appropriately when stimulated with CMV viral lysates, suggesting that the loss of alloreactivity was not due to global anergy of residual CD8+ T cells (data not shown). A similar loss of functional reactivity of CMV-specific CD8+ T cells was seen after depletion of CD4hiCD38+ T cells following stimulation with CMV viral lysates (Figure 3B). These data, in aggregate, confirm that alloreactive CD4+ T cells are the primary cells responding in the MLR and that the selective depletion of alloreactive CD4 T-cell clones abrogates alloreactivity following secondary challenge in the residual, unsorted CD8+ T-cell population.
Aggregate data from depletion of alloreactive CD4+ T cells from 5 donors and depletion of CD4hiCD38+ T cells following CMV lysate stimulation in 4 donors confirmed these results (Figure 3C). Following initial allogeneic stimulation and depletion of alloreactive CD4hiCD38+ T cells, alloreactivity in CD4+ and CD8+ T cells upon restimulation was not significantly different than that following autologous restimulation. Similarly, depletion of CMV-reactive CD4hiCD38+ T cells resulted in the loss of functional CD4+ and CD8+ T-cell reactivity to a level statistically indistinguishable from that seen after autologous stimulation (Figure 3C).
The residual T-cell receptor (TCR) repertoire following depletion of alloreactivity is diverse. In the experiments described in Figure 3, we established that depletion of CD4hiCD38+ T cells following alloantigen stimulation resulted in a residual population of PBMCs that remained capable of responding to viral antigens but was unable to respond to stimulation with the original alloantigenic stimulus. While we suspected that the ability to retain responsiveness to CMV was indicative of a diverse residual TCR repertoire, we proved this formally by analyzing diversity using molecular spectratyping. We amplified the CDR3 regions in 23 TCR Vβ subsets by PCR and subjected the resulting PCR products to electropheresis and quantitative densitometry to assess fragment length diversity of the CDR3 region within each TCR Vβ family.14 Previously, spectratyping has been used to assess the overall diversity within the human TCR repertoire, while qualitative changes in TCR diversity by spectratyping have been used to measure immune reconstitution in humans.20 In multiple CMV-seropositive subjects, we initially stimulated T cells with both CMV lysates and pooled alloantigenic PBMCs. Following each stimulus, we purified populations of CD4hiCD38+ and CD4intCD38– T cells by cell sorting and analyzed the TCR Vβ diversity within each population by spectratyping (Figure 4).
We found that the CD4hiCD38+ population obtained after CMV stimulation was the most restricted, with many TCR Vβ subsets demonstrating repertoire restriction consistent with one or several clones responding to CMV. In contrast, the CD4hiCD38+ population isolated following alloantigen stimulation was somewhat skewed but more diverse, consistent with a larger number of clones capable of responding to alloantigens (Figure 4A). The CD4intCD38– T-cell population, isolated following either CMV stimulation or stimulation using the alloantigen pool, was extremely diverse, as indicated by the Gaussian-appearing distribution of CDR3 fragment lengths within all TCR Vβ loci examined (Figure 4B). These data demonstrate that following depletion of alloantigen-specific T cells, we preserved a T-cell population not only capable of responding to viral antigens but containing a broad TCR repertoire, reflecting the specificity of our depletion method.
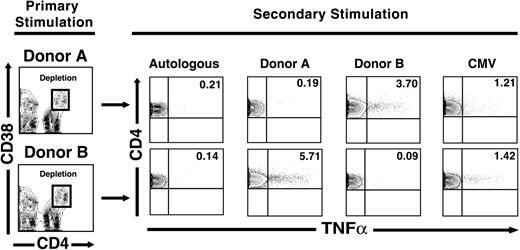
Depletion of alloreactive T cells following stimulation using cryopreserved apheresis products preserves viral and third-party alloantigen responses. In the experiments described in Figures 1, 2, 3, 4, we stimulated freshly obtained healthy donor PBMCs with pools of cells from 10 allogeneic donors. The clinical application of alloreactive T-cell depletion, in contrast, will likely require the use of cryopreserved PBMC products used as stimulators in one-way MLR reactions to deplete specific alloreactivity against a single HLA-mismatched recipient. To determine the feasibility of this approach in a preclinical setting, we obtained residual cryopreserved apheresis products collected routinely in a clinical cellular processing laboratory. Products from 2 HLA-mismatched donors that were CMV-seropositive (donors A and B) were used to stimulate cells from a healthy donor (donor C) in parallel one-way MLR stimulations (C → A and C → B). Following 7 days of stimulation, we depleted CD4hiCD38+ T cells by sorting and then restimulated residual cells with the primary stimulus, autologous cells (C → A/C → C) and cells from the donor not used in the initial stimulation (C → A/C → B). Following depletion of alloantigen-activated CD4hiCD38+ T cells, repeat stimulation with the original donor resulted in no CD4+ T-cell responsiveness above that seen following restimulation with autologous PBMCs (0.19% for C → A/C → A versus 0.21% for C → A/C → C; 0.09% C → B/C → B versus 0.14% for C → B/C → C; Figure 5). Consistent with prior results, we also saw abrogation of alloreactivity within the nondepleted residual CD8+ T-cell population, with reduction of the alloresponses in this T-cell subset to that seen in cells restimulated with autologous cells (data not shown). However, residual cells retained the ability to respond to a third-party stimulus as indicated by a significant frequency of responsive CD4+ T cells (3.7% for C → A/C → B and 5.71% for C → B/C → A; Figure 5) and to CMV antigens (1.21% for C → A/C → CMV and 1.42% for C → B/C → CMV; Figure 5). As expected, nondepleted CD8+ T cells also retained the capacity to respond to both alloantigens and to CMV antigens (data not shown). These data indicated that earlier methods, developed using pooled allogeneic PBMC as stimulators, were similarly effective when clinically obtained apheresis products were used instead.
Third-party alloreactivity is preserved after depletion of alloreactive cells stimulated with single-donor apheresis products. PBMCs from a healthy donor were stimulated with irradiated PBMCs obtained from clinically harvested apheresis products from 2 donors (designated A and B). Following 7 days of stimulation, the CD4hiCD38+ T-cell population was depleted using the gating strategy shown in the panels on the left. After depletion of alloantigen-specific CD4hiCD38+ T cells, repeat stimulation with the original donor resulted in activation of CD4+ T cells similar to that seen following autologous restimulation. However, residual cells remained capable of responding to either a third-party donor (eg, 3.7% of CD4+ T cells still responded functionally to donor B, and 1.21% of CD4+ T cells responded to CMV stimulation following depletion of cells originally stimulated with donor A, top). Results are representative of 2 similar experiments from healthy donors.
Third-party alloreactivity is preserved after depletion of alloreactive cells stimulated with single-donor apheresis products. PBMCs from a healthy donor were stimulated with irradiated PBMCs obtained from clinically harvested apheresis products from 2 donors (designated A and B). Following 7 days of stimulation, the CD4hiCD38+ T-cell population was depleted using the gating strategy shown in the panels on the left. After depletion of alloantigen-specific CD4hiCD38+ T cells, repeat stimulation with the original donor resulted in activation of CD4+ T cells similar to that seen following autologous restimulation. However, residual cells remained capable of responding to either a third-party donor (eg, 3.7% of CD4+ T cells still responded functionally to donor B, and 1.21% of CD4+ T cells responded to CMV stimulation following depletion of cells originally stimulated with donor A, top). Results are representative of 2 similar experiments from healthy donors.
Discussion
The results of our studies demonstrate that CFCs may be used to assess the frequencies of CD4+ and CD8+ T cells responding to alloantigen stimulation, leading to a more complete phenotypic characterization of alloreactive T cells in humans. The use of CFCs allowed us to study the functional characteristics of individual cells responding to stimulation in the MLR, leading us to conclude that (1) the bulk of human alloreactivity in the in vitro MLR resides in the CD4+ T-cell population; (2) the kinetics of alloantigen activation of T cells in the CFC assay are consistent with prior results using lymphocyte proliferation responses to assess alloreactivity; (3) CD4 up-regulation is a hallmark of chronic activation following either alloantigen or CMV stimulation; and (4) within the CD4+ T-cell subset, proliferation and the expression of secondary activation markers are restricted exclusively to CD4+ T cells expressing high levels of surface CD4 (CD4hi cells). Our findings regarding CD4 up-regulation are consistent with prior reports demonstrating that murine CD4+ T cells up-regulated levels of CD4+ following recall antigen20 or alloantigen challenge, 21 and with the observation that activated influenza-specific human CD4+ T cells identified by class II HLA-peptide tetramer staining were contained within the CD4hi population.22
We then stimulated cells with either viral antigens or pooled alloantigenic stimulator cells and subsequently depleted cells with the activated CD4hiCD38+ phenotype. Further experiments demonstrated that the residual PBMCs lacked the ability to respond to the initial stimulus but retained the ability to respond to alloantigens or viral antigens in cells stimulated with CMV or alloantigens, respectively, and retained a diverse TCR repertoire. Finally, we applied these methods to clinically obtained apheresis products, resulting in the depletion of specific alloreactivity in a one-way MLR with the preservation of CMV-specific T-cell responses and T cells capable of responding to third-party alloantigens.
Other investigators have developed alternate approaches to deplete23-31 or anergize32 alloreactive T cells. In one general strategy, cells expressing the CD25 antigen associated with the interleukin-2 receptor are depleted using a toxin-conjugated monoclonal antibody following the stimulation of donor cells with alloantigenic stimulators. In contrast to some previously described approaches, our method uses readily available fluorochrome-conjugated MAbs specific for easily identified surface markers. The use of 2 markers in combination greatly enhances the ability to discriminate activated and resting cells by flow cytometry. There is also a historic precedent for the use of sorted or otherwise separated cellular populations in clinical use, and such methods have proven safe in humans. Recent clinical studies have demonstrated that the infusion of relatively modest numbers of polyclonal CMV-specific T cells (ie, < 105/kg) may be sufficient to limit viral reactivation after allogeneic SCT.33 The degree of allodepletion we achieved (≥ 1-2 logs) was similar to that obtained using immunotoxin-based purging or through ex vivo approaches designed to induce anergy of alloreactive T cells.26,27,30,32 Consequently, we believe that standard apheresis products from healthy donors should yield sufficient quantities of alloreactivity-depleted T cells for clinical use, even if we assume that the precursor frequencies of alloreactive T cells in the haploidentical or mismatched setting may be significantly lower than those observed here. Given the demonstrated breadth of TCR diversity in the residual T-cell population following depletion of alloreactivity, relatively modest numbers of these cells (eg, 104-106/kg) might be sufficient to transfer meaningful infectious immunity without the need for prolonged cell sorting of the entire lymphoid fraction of an apheresis product.
Our results also suggest that alloreactivity in the CD8+ T-cell population is abrogated by the depletion of alloantigen-stimulated CD4+ T cells. Indeed, while a significant fraction of CD8+ T cells also expressed activation markers and produced cytokines following primary allogeneic stimulation, these cells were not capable of effector cytokine production if alloreactive CD4hiCD38+ T cells were first depleted prior to restimulation with alloantigens. These data are consistent with murine data that suggest that CD4+ T-cell help is more critical to the functional reactivation of CD8+ T cells than for the development of a primary response.34 While these data suggest that the depletion of alloreactive CD4+ T cells alone may be enough to lessen the risk of GVHD in the mismatched setting, clinical studies in humans will be required to assess whether the depletion of activation-marker–expressing CD8+ T cells will further decrease the risk of GVHD and whether the elimination of this T-cell subset might affect graft-versus-malignancy effects. Ideally, if unsorted CD8+ T cells could be safely infused in a cellular product depleted of alloreactive CD4+ T cells, CD8+ T cells specific for cancer-associated antigens35 would be preserved, increasing the likelihood that GVHD risk might be alleviated while sparing T cells important in preventing relapse. Indeed, in a recent trial wherein total CD8+ T cells were depleted from allogeneic donor grafts, a higher incidence of peri-engraftment fever and rash along with a similar rate of noncutaneous grade 2 to 4 GVHD were observed, relative to nondepleted controls.36 In another study characterizing post-SCT clonal T-cell expansions, CD4+ donor lymphocyte infusion was found to elicit a potent allogeneic response mediated by expanded alloreactive CD8+ T cells.37 In aggregate, these data support the notion that the selective depletion of alloreactive donor CD4+ T cells will likely reduce GVHD by limiting responses mediated both by CD4+ and CD8+ T cells recognizing recipient antigens.
The studies presented in this report are clearly at a preclinical stage. Additional studies in haploidentical donors and recipients and more closely matched pairs are needed. We also will need to confirm the consistency of this approach under conditions approximating those in a clinical cell-processing laboratory. At this time, MAbs to CD4 and CD38 expressly approved for clinical use are not available; however, research-grade MAbs with the appropriate infectious screening may be acceptable for phase 1/phase 2 trials and are less likely to mediate toxicity than immunotoxin conjugates.23,27,30 Similarly, reagents used in other preclinical approaches (eg, CFSE31 ) may be toxic to T cells at higher concentrations, potentially affecting the safety of such methods in humans. However, the growing body of clinical experience using MAbs and the increasing availability of high-speed sorting suggest this approach as a credible alternative to existing methods.
Additional studies also will be required to determine the frequencies of alloreactive CD4+ and CD8+ T cells that may be depleted in the setting of HLA-matched transplantation, given the likelihood that such frequencies are likely to be far lower than in the mismatched setting examined in the current studies. Even if this approach may not be extended to the setting of HLA-matched siblings, the potential to reduce the risk of GVHD in the setting of mismatched and haploidentical transplantation while sparing beneficial T-cell subsets may extend the benefits of transplantation to many subjects who are excluded from candidacy. These include older recipients at higher risk of GVHD than younger recipients and recipients lacking suitably matched sibling or registry donors. Finally, we believe that CFC-based assessment of alloreactivity may have independent clinical value as a marker of the risk for GVHD and as a measure of the success of clinical strategies to eliminate GVHD-mediating T cells in vitro and in human subjects.
Prepublished online as Blood First Edition Paper, July 29, 2004; DOI 10.1182/blood-2004-05-1918.
Supported in part by grants from the Gillson Longenbaugh Foundation (K.V.K.) and the National Institutes of Health (RO1 CA81247) (J.J.M.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Karen Ramirez for assistance with sorting; Pariya Sukhumulchandra for assistance with spectratyping; Susan Bryan for technical assistance; Safa Karandish and Elizabeth Shpall for assistance with apheresis samples; and Mike McCune for thoughtful review of the manuscript.