Abstract
In this paper, we show that plasma from patients with severe sepsis and septic shock but not normal plasma supports lipopolysaccharide (LPS) activation of epithelial cells expressing Toll-like receptor 4 (TLR4). Recombinant soluble myeloid differentiation protein-2 (MD-2) complemented normal plasma and allowed LPS activation of epithelial cells to levels measured with “septic” plasma, whereas soluble MD-2-depleted plasma lost its effects. The same “MD-2 activity” was found in urine from a patient with septic shock and in lung edema fluids from patients with adult respiratory distress syndrome (ARDS). Recombinant soluble MD-2 enabled LPS-dependent activation of epithelial cells bearing TLR4. LPS-binding protein (LBP) and soluble CD14 increased the sensitivity of TLR4-expressing epithelial cells to LPS but were not able to mediate LPS activation of these cells in the absence of soluble MD-2. An anti-MD-2 monoclonal antibody blocked LPS activation of TLR4-expressing cells only in the presence of septic plasma or septic urine. These results suggest that septic plasma containing soluble MD-2 leaking into the extravascular space supports LPS activation of TLR4-expressing epithelial cells. We therefore propose that soluble MD-2 is an important mediator of organ inflammation during sepsis. (Blood. 2004;104:4071-4079)
Introduction
Human sepsis accounts for more than 200 000 deaths per year in the United States1 and results from a dysregulation of innate responses to bacterial pathogens.2,3 Septic shock, its most severe form, is characterized by a profound vasodilation and extravascular plasma leakage resulting from an increase in endothelial permeability.4 Gram-negative lipopolysaccharide (LPS) triggers host responses similar to those seen during human septic shock and has served for several decades as a useful in vivo and in vitro model molecule.5 Several host humoral and cell-surface proteins participate in the innate recognition of LPS, including the acute-phase LPS-binding protein (LBP), and the glycoproteins CD14, Toll-like receptor 4 (TLR4), and myeloid differentiation protein-2 (MD-2).6 In myeloid cells, the LPS receptor complex is made of CD14, TLR4, and MD-2, whereas in endothelial cells only TLR4 and MD-2 are expressed.6 LPS responses of endothelial cells require the presentation of LPS to the TLR4/MD-2 membrane receptor by LBP and soluble CD14.7 Epithelial cells may only express TLR4, and the cofactor requirement of these cells for LPS responses remains to be defined.8-10
MD-2 is produced by several cell types as a 20- to 25-kDa glycoprotein, binds to LPS, and confers LPS responsiveness to TLR4-expressing cells.11-19 Some of the MD-2 molecules remain attached to TLR4, whereas the rest is secreted in the extracellular milieu.7,20 MD-2 seems to be essential in mouse cells to bring TLR4 from the Golgi to the cell surface.21 Cystein residues, N-glycosylation, and oligomerization are important for MD-2 function.22-26 MD-2 knock-out mice are resistant to LPS-induced lethality,21 a phenotype very similar to that observed in mice lacking TLR4, CD14, or LBP.27-29 However, little is known about the function of soluble MD-2 with regard to LPS activation of cells and as a potential mediator of septic shock.7,20,26
In this paper, we propose that soluble MD-2 in plasma from patients with severe sepsis and septic shock is an important cofactor for LPS activation of TLR4-expressing cells. Plasma LBP and soluble CD14 also play a role, most probably by shuttling LPS to MD-2 or the MD-2/TLR4 receptor complex. Soluble MD-2 is required for LPS activation of epithelial cells expressing TLR4, whereas it down-regulates LPS activation of monocytic and endothelial cells expressing both TLR4 and MD-2 at the cell surface, most probably by trapping LPS in solution. This supports a novel concept that soluble MD-2 is a modulator of LPS activation of cells with opposing effects in the circulation and in the extravascular space during sepsis.
Patients, materials, and methods
Patients and samples of plasma and edema fluid
Patients meeting the criteria for severe sepsis and septic shock were prospectively enrolled in the study.30 The research protocol was approved by the University Hospital of Geneva's Ethic Committee. Informed consent was provided according to the Declaration of Helsinki. Demographic, microbiologic, and outcome data were recorded. Whole heparinized blood was drawn within 48 hours after the admission to the intensive care unit (ICU). Plasma C-reactive protein levels were measured in our clinical laboratory facility. In some patients, additional samples were obtained at different times during the ICU stay. Platelet-poor plasma was immediately collected by centrifugation at 4°C, divided into aliquots, and kept frozen at -70°C until the day of the assay. Plasma samples were obtained using the same protocol in healthy human volunteers. To compare data from septic patients with data of a population of patients with a noninfectious systemic inflammatory disease, we obtained 19 sera from patients with an acute exacerbation of rheumatoid arthritis (kindly provided to us by C. Gabay, University Hospital of Geneva). One urine sample (1600 mL) was collected during the first 24 hours of the ICU stay of a patient with septic shock resulting from a Staphylococcus aureus myositis. Paired plasma and lung edema fluid samples were obtained in 4 septic patients within a 24-hour period following intubation and mechanical ventilation for acute respiratory distress syndrome (ARDS) and in 2 patients with congestive heart failure, as described elsewhere.31
Cells
Human embryonic kidney 293 (HEK293) cells (obtained from D. Trono, University of Geneva, Switzerland) and the human liver HepG2 cell line (American Type Culture Collection [ATCC], Rockville, MD) were stably transfected with Flag-tagged human TLR4 cDNA (donated by M. Rothe, Tularik, South San Francisco, CA; TLR4-HEK293 cells). HEK293 cells were stably transduced with both Flag-tagged human TLR4 cDNA and Flag/6xHis-tagged MD-2 cDNA (obtained from K. Miyake, Saga University, Japan; TLR4/MD2HEK293 cells). Chinese hamster ovary (CHO) cells (ATCC) were stably transduced with both C-myc-tagged human TLR4 cDNA and C-myc/Protein C-tagged MD-2 cDNA. cDNAs were subcloned into pCDNA 3.1 plasmids (Invitrogen, San Diego, CA) containing neomycin or hygromycin resistance genes. Transfectants were selected by using these antibiotics and cell sorting by flow cytometry using anti-TLR4 (eBioscience, San Diego, CA) and anti-6xHis (Sigma, St Louis, MO) or anti-C-myc (9E10; ATCC) antibodies. Human MD-2 cDNA was also subcloned into a bicistronic vector coexpressing Flag/6xHis-tagged MD-2 and the green fluorescent protein (GFP). This vector served for stable transfection of HEK293 cells, HeLa cells (ATCC), and CHO cells. Cells expressing high levels of MD-2 were selected by successive cell sorting based on the high GFP expression and adapted to serum-free culture (SFM II media; Gibco, Grand Island, NY). The Sf9 (Spodoptera frugiperda 9) insect cell line was used to produce both soluble MD-2 and the chimeric TLR4/MD-2 fusion protein by infection with recombinant baculovirus containing the appropriate cDNA. The human promonocytic THP-1 cells were obtained from the ATCC and were differentiated with 10-7 M calcitriol (1,25 dihydroxy-vitamin D3; Roche, Basel, Switzerland) for 3 days before the assay. Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords by classic collagenase digestion and provided to us by F. Mach, University of Geneva.
Purification of soluble MD-2 and chimeric TLR4/MD-2 fusion protein
Flag/6xHis-tagged soluble MD-2 was purified from serum-free supernatants of HEK293 cells expressing high levels of MD-2 or from supernatants of Sf9 cells infected with baculovirus containing the MD-2 cDNA using a Ni+-NTA (nitrilotriacetic acid) agarose column (QIAGEN, Hilden, Germany) and an anti-FLAG M2 mAb (monoclonal antibody) affinity matrix (Sigma). Supernatants from HEK293, HeLa, and CHO cells expressing high levels of Flag/6xHis-tagged MD-2 and containing 10% fetal calf serum (FCS) were also collected and kept frozen at -20°C. Supernatants from untransfected HEK293 cells served as control.
To generate the recombinant TLR4/MD-2 chimeric protein, cDNA encoding the extracellular portion of human TLR4 linked to MD-2 via a glycine serine (GGGGS3) linker was assembled by using polymerase chain reaction (PCR). FLAG and 6xHIS tags were included at the C-terminus of MD-2 for detection and purification purposes. The cDNA cassette was cloned into the baculovirus expression vector pFASTBAC1 (Invitrogen) and subsequently inserted into bacmid DNA by homologous recombination. Following generation of a viral stock, Sf9 cells were superinfected. Forty-eight hours later, the recombinant fusion protein was purified from cell lysates by using an anti-FLAG M2 mAb affinity matrix (Sigma).
Production of an anti-MD-2 monoclonal antibody
Eight-week-old female BALB/c mice (IFFA CREDO, Les Oncins, France) were immunized with a subcutaneous injection of 106 TLR4/MD-2-expressing CHO cells/mL in RIBI adjuvant (Sigma) at days 0, 7, and 28 as previously described.32 Mice having specific TLR4/MD-2 serum antibodies were “hyperboosted” subcutaneously with the chimeric TLR4/MD-2 fusion protein either 3 or 4 days prior to fusion. Draining lymph node B cells were fused with the mouse myeloma cell line P3-X63-Ag8.653. B-cell extraction and cellular fusions were performed as previously described.32 Cells were plated at a concentration of 104 myeloma cells/well and grown for 10 to 14 days in culture medium supplemented with histone acetyltransferase (HAT; Sigma). Supernatants from wells containing viable hybridoma cells were screened on mock-transfected CHO cells versus TLR4/MD-2-transfected CHO cells for TLR4/MD-2 specificity by fluorescence activated cell sorting (FACS) analysis. Cells were then incubated with supernatant and an allophycocyanin (APC)-coupled goat-antimouse immunoglobulin G (IgG) antibody (Molecular Probes, Leiden, the Netherlands), and analyzed on a FACScalibur (Becton Dickinson, Vienna, Austria) in the FL-4 channel.
To determine mAb specificity, HEK293 cells were plated in 6-well plates at a density of 2.5 × 105 cells/well in 2 mL culture medium containing 10% FCS. Sixteen hours after plating, cells were transfected with 0.75 μg appropriate vector(s) using Fugene reagent (Roche) according to the manufacturer's guidelines. Rabbit orthologs of TLR4 and MD-2 were cloned by reverse transcriptase (RT)-PCR from rabbit peripheral blood lymphocyte (PBL) first-strand cDNA by RT-PCR using degenerate primers. Two days after transfection, cells were stained with the appropriate monoclonal antibody and an APC-coupled goat antimouse IgG antibody and analyzed using the FACScalibur.
Cell stimulation assays
TLR4-HEK293 cells expressing more than 90% surface TLR4 were used for the stimulation assays. On the day of the assay, TLR4-HEK293 cells were collected by trypsin treatment, resuspended in Dulbecco modified Eagle medium (DMEM; Gibco) containing 2% FCS and distributed into 96-well plates at the concentration of 60 000 cells per well. Plasma and lung edema fluid from patients and plasma from volunteers was added at the final concentration of 5%. Escherichia coli K12LCD25 LPS (List; Alexis, Lausen, Switzerland) was added to the wells at the final concentration of 10 ng/mL and incubated at 37°C for 21 hours in a 5% CO2-enriched atmosphere. In some experiments LPS was substituted for 10 ng/mL recombinant human interleukin 1β (IL-1β; a gift from J.-M. Dayer, University of Geneva, Switzerland). Conditioned supernatants were then collected and assayed for human IL-8 protein by enzyme-linked immunosorbent assay (ELISA) as described.33 In some experiments, TLR4-HEK293 cells were substituted for HUVECs, calcitriol-differentiated THP-1 cells, or HEK293 cells expressing both MD-2 and TLR4. THP-1 cells (ATCC) were cultured in RPMI 1640 containing 10% FCS in suspension for 3 days with 10-7 M calcitriol. In blocking experiments, we used anti-human LBP 2B5, anti-human CD14 28C5 mAbs (provided by P. S. Tobias, The Scripps Research Institute, La Jolla, CA), an anti-human TLR4 HTA125 (eBioscience), and the anti-human MD-2 18H10 at 20, 2, 2, and 2 μg/mL final concentration, respectively. TLR4-HEK293 cells were also incubated with normal versus septic plasma supplemented with 1 μg/mL recombinant human LBP, 200 ng/mL recombinant soluble CD14, or both. These concentrations of LBP and sCD14 correspond to levels found in 5% of “acute-phase plasma.” In one experiment, TLR4-HEK293 cells were transiently transfected the day before the assay with a pGL2 vector containing the firefly luciferase reporter gene under the control of a minimal, nuclear factor κB (NF-κB)-dependent, E-selectin promoter (a gift from D. Trono). Cells were cotransfected with a pRL vector containing the Renilla luciferase gene driven by a constitutive thymidine kinase promoter (Promega, Madison, WI). Cells were then stimulated with LPS in the presence of normal plasma, septic plasma, or soluble MD-2 for 6 hours. Luciferase activity was determined by incubation of the cell lysates with the dual luciferase substrates in a luminometer. Data were expressed as relative light intensity ratio of firefly/Renilla luciferase activity. In other experiments, leukocytes from healthy volunteers were exposed to heterologous plasma from patients with sepsis. Heparinized blood from a healthy subject was centrifuged, and plasma was removed. Blood cells were resuspended in RPMI 1640 containing 25% plasma from patients with severe sepsis or septic shock or from volunteers. After 6 hours of incubation at 37°C, conditioned supernatants were collected and assayed for IL-8 and tumor necrosis factor α (TNF-α) by ELISA (Endogen, Woburn, MA). In one experiment, IL-8 was measured in the conditioned supernatant of HepG2 cells stimulated with soluble peptidoglycan from Bacillus subtilis and S aureus (Sigma) in the presence of normal or septic plasma, or in the presence of soluble MD-2.
Transfer of soluble MD-2 to TLR4-expressing cells
TLR4-HEK293 cells were incubated with DMEM containing 10% plasma from healthy volunteers, from patients with septic shock, or with 1:2 dilution of conditioned supernatants of HEK-293 cells expressing recombinant soluble human MD-2. Cells were then washed in pH 7.2 phosphate-buffered saline (PBS) and stained with the anti-human MD-2 18H10 mAb (4 μg/mL) and an APC-conjugated goat anti-mouse IgG. Mean fluorescence index was determined by using the FACScalibur.
Soluble MD-2 depletion from septic plasma
TLR4-HEK293 cells were used to affinity deplete soluble MD-2 contained in septic plasma. TLR4-HEK293 cells or “mock” HEK-293 cells (7 × 107) transfected with an empty vector were collected by using PBS-EDTA (ethylenediaminetetraacetic acid) 5 mM, washed with DMEM, pelleted, and incubated on ice for 2 hours with 550 μL 10% plasma from a patient with septic shock. Plasma samples were then collected by centrifugation and used to test the capacity of TLR4- versus mock-depleted plasma to support anti-MD-2 antibody binding to new TLR4-HEK293 cells. Depleted plasma samples were also used to test whether they supported LPS-dependent activation of TLR4-HEK293 cells.
LPS binding to cells
Fluorescein isothiocyanate (FITC)-conjugated Re595 LPS (1 μg/mL; kindly provided by P. S. Tobias, The Scripps Research Institute) was incubated in RPMI supplemented with 10% septic plasma, 10% normal plasma, or supernatant from HEK293 cells transfected with human MD-2 containing 10% FCS, with 3 × 105 TLR4-HEK293 cells in suspension for 0, 5, and 15 minutes. Cells were then put on ice and washed, and the percentage of positive cells was detected by FACS.
Quantitative PCR
Circulating monocytes from healthy volunteers and patients with severe sepsis and septic shock were purified by using a classic Ficoll-paque gradient followed by a 45-minute plastic adherence protocol. Adherent monocytes (> 95% purity) were then lyzed in Trizol (Gibco), and total RNA was extracted by using chloroform/ethanol. Complementary DNA was obtained by reverse transcription (reverse transcriptase; Roche, Mannheim, Germany) using random hexamers. Levels of MD-2 mRNA were determined by TaqMan PCR using a fluorescent probe for MD-2, AAAGCGCAAAGAAGTTATTTGCCGAGGA, and 2 primers, sense 5′-CTCTATATAACTGTCAACACCATGAATCTTC and antisense 5′-TCTGCAAAAAGAGTAATCGTCATCA. As a control we co-amplified the 18S ribosomal RNA by using a fluorescent probe and a primer mix from Perkin Elmer (Boston, MA). Cycle thresholds (Ct) were determined for both MD-2 and 18S in samples and normalized with a known calibrator sample (human monocyte cDNA). Levels of MD-2 mRNA were determined by using the comparative method as proposed by the manufacturer (Perkin Elmer) and expressed as arbitrary units.
Statistical analysis
Data were expressed as median (range). Comparisons between groups were done using a Mann-Whitney U test (2 groups) and using a one-way analysis of variance (ANOVA) for more than 2 groups (Statview software, SAS Institute, Cary, NC).
Results
Patients
Plasma samples were obtained within 48 hours after the admission to the medical ICU in 15 patients with severe sepsis (10 men, 5 women; mean age ± 1 SD, 54 ± 18 years) and 26 patients with septic shock (13 men, 13 women; 61 ± 17 years). The ICU mortality was 33% in patients with severe sepsis and 23% in those with septic shock. The main infectious source was severe community acquired pneumonia (n = 19). Other sources were nosocomial pneumonia (n = 4), bacterial meningitis (n = 4), abdominal sepsis (n = 3), urosepsis (n = 3), primary bacteremia (n = 2), bacterial endocarditis (n = 2), myositis (n = 1), and osteomyelitis (n = 1). Bacterial pathogens could be isolated in 8 patients with severe sepsis (3 S aureus, 1 Streptococcus pneumoniae, 1 Legionella pneumophila, and 1 Pseudomonas aeruginosa) and in 19 patients with septic shock (7 E coli,5 S pneumoniae,2 S aureus,2 Neisseria meningitidis, 1 L pneumophila, and 1 Haemophilus influenzae). The mean plasma C-reactive protein levels ± 1 SD were 213 ± 109 mg/L in patients with severe sepsis (range, 66-436 mg/L) and 283 ± 116 mg/L in patients with septic shock (range, 120-530 mg/L). Plasma samples were obtained in 19 patients presenting with an acute exacerbation of rheumatoid arthritis (8 men, 11 women; mean age ± 1 SD, 50 ± 13 years). Their plasma C-reactive protein was 50 ± 13 mg/L (mean ± 1 SD). Plasma was also obtained in 12 healthy volunteers (mean age ± 1SD, 39 ± 10 year; all had plasma C-reactive protein < 5 mg/L). One urine sample (1600 mL) was collected during the first 24 hours of the ICU stay of a patient with septic shock because of an S aureus myositis.
Effect of plasma on LPS activation of TLR4-expressing cells
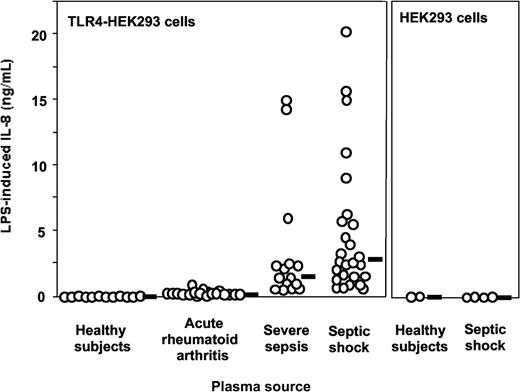
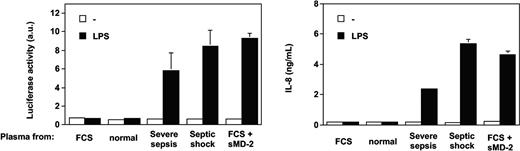
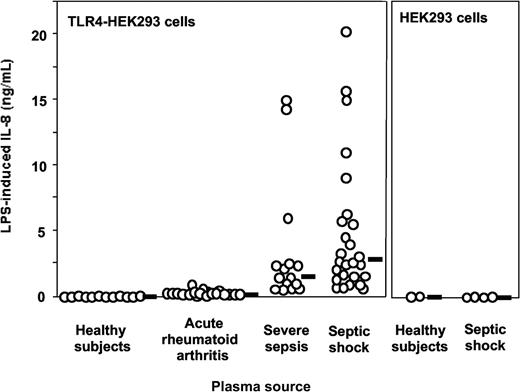
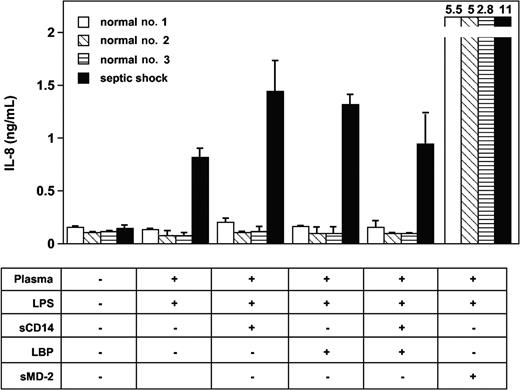
Plasma from patients with severe sepsis and septic shock collected within 48 hours after ICU admission supported LPS activation of TLR4-HEK293 cells but not plasma from healthy volunteers (Figure 1, shown with 5% plasma concentrations). The median (range) levels of LPS-induced IL-8 were healthy volunteers, 0 ng/mL (0-0.07 ng/mL); acute rheumatoid arthritis, 0.25 ng/mL (0.1-0.95 ng/mL); severe sepsis, 1.45 ng/mL (0.29-14.99 ng/mL); and septic shock, 2.68 ng/mL (0.61-20.3 ng/mL). No activation was observed in the absence of LPS. This effect was dependent on TLR4 expression because HEK293 cells transfected with an empty vector did not respond to LPS plus septic plasma (Figure 1). Similar results were obtained when target cells were HepG2 cells transfected with TLR4 (data not shown). Plasma from 19 patients with acute exacerbation of rheumatoid arthritis supported only low levels of LPS stimulation in TLR4-HEK293 cells, levels that were not significantly different from those measured in normal plasma (Figure 1). Levels of activation were significantly higher in plasma from patients with severe sepsis and septic shock compared with plasma sampled in volunteers and in patients with rheumatoid arthritis (P < .001, one-way ANOVA). In 11 patients with severe sepsis and septic shock, additional plasma samples were obtained during their ICU stay (45 samples total, up to 36 days after ICU admission). All plasma samples supported significant LPS activation of TLR4-HEK293 cells, although there was a net tendency to decreased activity with time (data not shown). Plasma samples from patients with gram-negative, gram-positive infection, and in whom the pathogen could not be identified supported equally well the LPS activation of TLR4-HEK293 cells (P = NS). In contrast, LPS activation of TLR4-HEK293 cells was significantly increased with plasma from patients who eventually died compared with those who survived (median IL-8 levels, 9.1 ng/mL; range, 0.92-20.3 versus 1.9 ng/mL; range, 0.29-11, respectively; P = .008, Mann Whitney U test). The septic urine from 1 patient also supported LPS activation of TLR4-HEK293 cells (data not shown).
LPS activation of TLR4-transfected HEK293 cells (IL-8 secretion) is supported by plasma from patients with severe sepsis and septic shock but not by plasma from healthy volunteers. Healthy volunteers versus severe sepsis or septic shock, P < .001; acute rheumatoid arthritis versus severe sepsis or septic shock, P < .01; severe sepsis versus septic shock, P = NS, one-way ANOVA. Plasma from patients with acute exacerbation of rheumatoid arthritis only minimally supported LPS activation of TLR4-HEK293 cells (healthy volunteers versus acute rheumatoid arthritis, P = NS, one-way ANOVA). LPS did not activate nontransfected HEK293 cells in the presence of plasma. Plasma concentration used in this figure was 5%. Horizontal black bars represent the median.
LPS activation of TLR4-transfected HEK293 cells (IL-8 secretion) is supported by plasma from patients with severe sepsis and septic shock but not by plasma from healthy volunteers. Healthy volunteers versus severe sepsis or septic shock, P < .001; acute rheumatoid arthritis versus severe sepsis or septic shock, P < .01; severe sepsis versus septic shock, P = NS, one-way ANOVA. Plasma from patients with acute exacerbation of rheumatoid arthritis only minimally supported LPS activation of TLR4-HEK293 cells (healthy volunteers versus acute rheumatoid arthritis, P = NS, one-way ANOVA). LPS did not activate nontransfected HEK293 cells in the presence of plasma. Plasma concentration used in this figure was 5%. Horizontal black bars represent the median.
Septic plasma however had no “enhancing” effect in nontransfected HepG2 cells (naturally expressing TLR1, TLR2, and TLR6 but not TLR4) when these cells were stimulated with the TLR2 agonist soluble gram-positive peptidoglycan (not shown). Septic plasma had no effect when epithelial cells were stimulated with recombinant IL-1β either. This indicated that the septic plasma effect observed in TLR4-HEK293 cells and endothelial cells stimulated with LPS was not a general phenomenon but was rather specific for the interaction between LPS and TLR4.
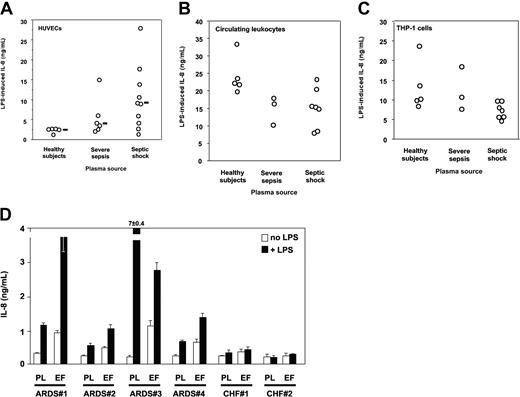
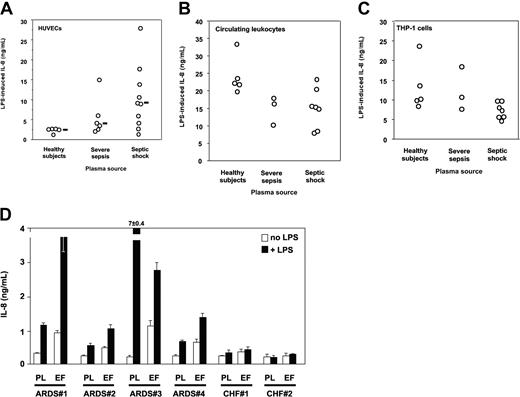
Plasma is required for HUVECs to respond to LPS.34 Plasma from septic patients was however markedly more potent in supporting LPS activation of HUVECs than normal plasma. Median LPS-induced IL-8 levels (range) were healthy volunteers, 2.74 ng/mL (1.4-2.77 ng/mL); severe sepsis, 3.96 ng/mL (2.17-15.13 ng/mL); and septic shock, 9.19 ng/mL (1.41-28 ng/mL) (healthy volunteers versus severe sepsis or septic shock, P < .05, one-way ANOVA; Figure 2A). Circulating leukocytes containing monocytes, polymorphonuclear neutrophils, and lymphocytes from healthy volunteers resuspended in heterologous plasma responded to LPS by secreting IL-8. In this case, plasma from septic patients did not increase cell responsiveness to LPS, but rather they tended to decrease it as compared with that of volunteers (P = NS; Figure 2B). Similar results were obtained when circulating leukocytes were substituted with calcitriol-differentiated THP-1 cells (Figure 2C) and when we measured TNF instead of IL-8 as a marker for cell activation (not shown). Lung edema fluid from septic patients supported LPS activation of TLR4-transfected HEK293 cells to levels similar to those seen with septic plasma (Figure 2D). In contrast, neither plasma nor edema fluid from patients with CHF did support LPS activation of TLR4-transfected cells. Taken together these results indicated that only plasma from patients with severe sepsis and septic shock supported activation of TLR4-expressing epithelial cells, and septic plasma induced a markedly higher LPS response in human endothelial cells than did normal plasma. Only lung edema fluid from patients with ARDS in a context of sepsis but not edema fluid from a noninflammatory origin (CHF) supported LPS activation of TLR4-HEK293 cells, most likely because septic samples contain soluble MD-2.
LPS activation of HUVECs, human circulating leukocytes, calcitriol-differentiated THP-1 cells, and TLR4-transfected HEK293 cells. (A) LPS activation (IL-8 secretion) of HUVECs is significantly increased by plasma from patients with severe sepsis and septic shock compared with plasma from healthy volunteers. Healthy volunteers versus severe sepsis or septic shock, P < .05, one-way ANOVA. Horizontal black bars represent the median. (B) LPS activation (IL-8 secretion) of human circulating leukocytes from healthy volunteers is decreased by plasma from patients with severe sepsis and septic shock compared with plasma from healthy volunteers (1 experiment of 3 similar experiments, P = NS between groups, one-way ANOVA). (C) LPS activation (IL-8 secretion) of calcitriol-differentiated THP-1 cells is decreased by plasma from patients with severe sepsis and septic shock compared with plasma from healthy volunteers (1 experiment of 3 similar experiments, P = NS between groups, one-way ANOVA). (D) LPS activation of TLR4-transfected HEK293 cells (IL-8 secretion) is supported by plasma from patients with ARDS with severe sepsis and septic shock and by undiluted lung edema fluids. In 4 septic patients with ARDS (patients no. 1-4) and in 2 patients with congestive heart failure (CHF; no. 1 and no. 2), plasma samples were taken at the same time as edema fluid. White bars indicate cell activation in the absence of LPS; black bars, cell activation in the presence of LPS; PL, plasma; EF, lung edema fluid. Mean ± 1 SD of triplicates, 1 representative experiment of 2 similar experiments. Neither plasma nor edema fluid supported LPS activation of untransfected HEK293 cells above basal levels (not shown).
LPS activation of HUVECs, human circulating leukocytes, calcitriol-differentiated THP-1 cells, and TLR4-transfected HEK293 cells. (A) LPS activation (IL-8 secretion) of HUVECs is significantly increased by plasma from patients with severe sepsis and septic shock compared with plasma from healthy volunteers. Healthy volunteers versus severe sepsis or septic shock, P < .05, one-way ANOVA. Horizontal black bars represent the median. (B) LPS activation (IL-8 secretion) of human circulating leukocytes from healthy volunteers is decreased by plasma from patients with severe sepsis and septic shock compared with plasma from healthy volunteers (1 experiment of 3 similar experiments, P = NS between groups, one-way ANOVA). (C) LPS activation (IL-8 secretion) of calcitriol-differentiated THP-1 cells is decreased by plasma from patients with severe sepsis and septic shock compared with plasma from healthy volunteers (1 experiment of 3 similar experiments, P = NS between groups, one-way ANOVA). (D) LPS activation of TLR4-transfected HEK293 cells (IL-8 secretion) is supported by plasma from patients with ARDS with severe sepsis and septic shock and by undiluted lung edema fluids. In 4 septic patients with ARDS (patients no. 1-4) and in 2 patients with congestive heart failure (CHF; no. 1 and no. 2), plasma samples were taken at the same time as edema fluid. White bars indicate cell activation in the absence of LPS; black bars, cell activation in the presence of LPS; PL, plasma; EF, lung edema fluid. Mean ± 1 SD of triplicates, 1 representative experiment of 2 similar experiments. Neither plasma nor edema fluid supported LPS activation of untransfected HEK293 cells above basal levels (not shown).
Involvement of LBP, soluble CD14, TLR4, and soluble MD-2
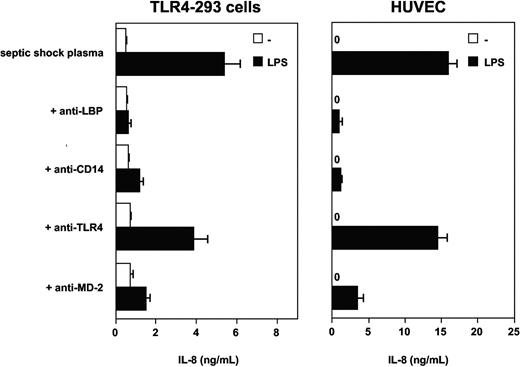
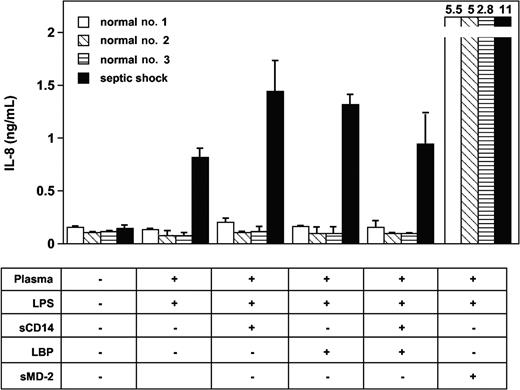
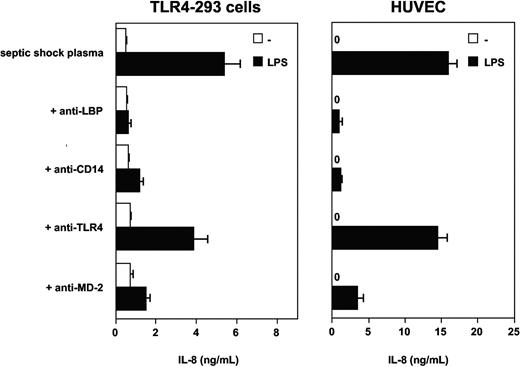
Experiments with blocking monoclonal antibodies to human LBP and soluble CD14 were then used to test the possible involvement of these plasma glycoproteins in the effect observed with septic plasma. This was motivated by the facts that both molecules have been implicated in the mediation of cellular LPS effects and both are found at elevated levels in plasma from patients with septic shock. Anti-LBP, anti-CD14, and anti-MD-2 mAbs had marked blocking effects on LPS-induced TLR4-HEK293 cell and HUVEC activation in the presence of septic plasma (Figure 3). The anti-TLR4 mAb HTA125 had only partial inhibitory effect (varying from 20% to 50% inhibition, depending on the experiments, n = 3). However, the addition of recombinant human LBP, recombinant soluble CD14, or both to normal plasma at acute-phase concentrations did not restore the activity found in plasma from septic patients (Figure 4). Concentrations of both proteins above and below the levels commonly measured in plasma from patients with sepsis were also incapable of inducing LPS activation of TLR4-HEK293 cells (not shown). The LBP used in these studies was shown to be active in supporting FITC LPS-binding to CD14-expressing cells, and soluble CD14 supported the LPS activation of endothelial cells, as described elsewhere.35 In 2 separate experiments, the addition of LBP and soluble CD14 to septic plasma consistently increased the LPS-dependent activation of TLR4-HEK293 cells (Figure 4, and data not shown).
Septic plasma-dependent LPS activation of TLR4-HEK293 cells is inhibited by monoclonal antibodies to LBP, CD14, TLR4, and MD-2. Mean ± 1 SD of triplicates. One representative experiment from 3 similar experiments.
Septic plasma-dependent LPS activation of TLR4-HEK293 cells is inhibited by monoclonal antibodies to LBP, CD14, TLR4, and MD-2. Mean ± 1 SD of triplicates. One representative experiment from 3 similar experiments.
Recombinant soluble MD-2 (sMD-2) added to normal plasma restores LPS activation of TLR4-HEK293 cells. The addition of LBP and soluble CD14 (sCD14) to normal plasma did not support LPS activation of TLR4-HEK293 cells. Mean ± 1 SD of triplicates. One representative experiment from 3 similar experiments.
Recombinant soluble MD-2 (sMD-2) added to normal plasma restores LPS activation of TLR4-HEK293 cells. The addition of LBP and soluble CD14 (sCD14) to normal plasma did not support LPS activation of TLR4-HEK293 cells. Mean ± 1 SD of triplicates. One representative experiment from 3 similar experiments.
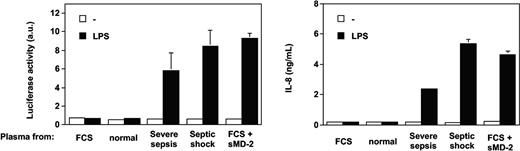
Only the addition of soluble MD-2 to normal plasma allowed TLR4-HEK293 cells to respond to LPS (Figure 4). Recombinant soluble MD-2 was produced by using different mammalian cell lines transfected with human MD-2 cDNA. Supernatants from HEK293, HeLa, and CHO cells transfected with MD-2 cDNA, containing 10% FCS, supported TLR4-HEK293 cell activation by LPS (data not shown). Partially purified soluble MD-2 from serum-free supernatant alone supported the LPS activation of TLR4-HEK293 cells, but this activation was amplified by the addition of recombinant LBP or FCS containing LBP and soluble CD14 (data not shown). Importantly, the effect observed with septic plasma or soluble MD-2 on LPS-treated TLR4-HEK293 cells could be reproduced by using an alternative (NF-κB-dependent cell activation read out), whereby the E-selectin minimal promoter drives expression of the luciferase gene (Figure 5).
Septic plasma and soluble MD-2 support LPS activation of TLR4-HEK293 cells in an NF-kappaB-dependent manner. Parallel activation of an endothelial leukocyte adhesion molecule (ELAM)-luciferase reporter gene and IL-8 production. Mean of triplicates ± 1 SD. One representative experiment from 2 similar experiments.
Septic plasma and soluble MD-2 support LPS activation of TLR4-HEK293 cells in an NF-kappaB-dependent manner. Parallel activation of an endothelial leukocyte adhesion molecule (ELAM)-luciferase reporter gene and IL-8 production. Mean of triplicates ± 1 SD. One representative experiment from 2 similar experiments.
Various plasma concentrations were used in the LPS stimulation experiments with TLR4-HEK293 cells described in Figure 1. These serial plasma dilutions indicated that the effect of septic plasma was linear between 1% and 10% for the vast majority of the plasma samples tested. The effect was found to be maximal between 10% and 20%, whereas concentrations above 20% consistently induced a decrease in the IL-8 signal, and almost no costimulatory effect was seen when plasma concentrations reached 80% to 90% (data not shown).
Although septic plasma induced a marked increase of LPS activation of HUVECs, we failed to demonstrate that soluble MD-2 played an enhancing role in this cell type. The addition of soluble MD-2 to normal plasma or to purified LBP and soluble CD14 did not increase LPS activation of HUVECs. The LPS activation-enhancing effect was down-regulated when high concentrations of septic plasma or exogenous soluble MD-2 were used. The septic plasma-dependent LPS activation of HUVECs could be blocked with anti-LBP and anti-CD14 mAbs and markedly decreased (> 70%) with an anti-MD-2 mAb (see below). The anti-MD-2 mAb blocked LPS activation of HUVECs through its interaction with membrane-bound MD-2.
Transfer of soluble MD-2 contained in septic plasma to TLR4
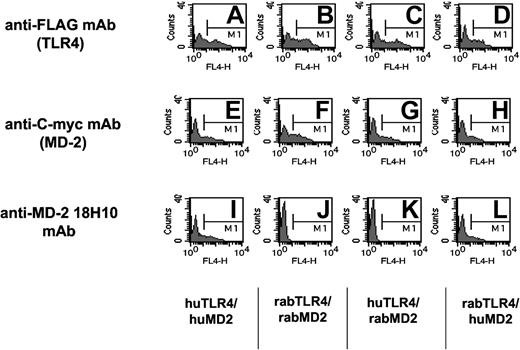
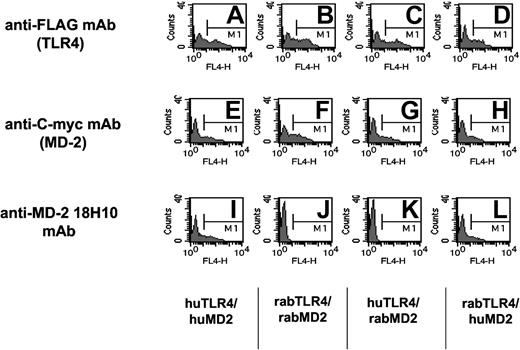
To further investigate the role of soluble MD-2 in plasma from septic patients, we have used a new mAb raised against cell surface human TLR4/MD-2-expressing cells (18H10 mAb). This antibody was found to recognize membrane-bound human MD-2, because it was labeled by FACS MD-2 transfectants coexpressing either the human or the rabbit TLR-4 receptor. It did not, however, bind rabbit MD-2 and did not recognize TLR4 transfectants (Figure 6).36 This mAb did not recognize MD-2 in its soluble form or in a solid-phase assay (ELISA). This antibody labeled HUVECs and monocytes but not lymphocytes. It blocks LPS activation of cells expressing TLR4/MD-2, such as monocytes, whole blood, or TLR4/MD-2 HEK293 cell transfectants (data not shown).
Anti-human MD-2 18H10 mAb binds to HEK293 cell transfectants expressing human MD-2 either with human TLR4 or rabbit TLR4, but not to rabbit MD-2. The specificity of the 18H10 antibody is shown by flow cytometry analysis of HEK293 cells transiently transfected with either human TLR4 and human MD-2 (A, E, and I), rabbit TLR4 and rabbit MD-2 (B, F, and J), human TLR4 and rabbit MD-2 (C, G, and K), or rabbit TLR4 and human MD-2 (D, H, and L). Cells were incubated with anti-FLAG mAb (to detect TLR4 expression), anti-C-myc mAb (to detect MD-2 expression), or the anti-human MD-2 18H10 mAb followed by an allophycocyanin (APC)-coupled anti-mouse IgG antibody.
Anti-human MD-2 18H10 mAb binds to HEK293 cell transfectants expressing human MD-2 either with human TLR4 or rabbit TLR4, but not to rabbit MD-2. The specificity of the 18H10 antibody is shown by flow cytometry analysis of HEK293 cells transiently transfected with either human TLR4 and human MD-2 (A, E, and I), rabbit TLR4 and rabbit MD-2 (B, F, and J), human TLR4 and rabbit MD-2 (C, G, and K), or rabbit TLR4 and human MD-2 (D, H, and L). Cells were incubated with anti-FLAG mAb (to detect TLR4 expression), anti-C-myc mAb (to detect MD-2 expression), or the anti-human MD-2 18H10 mAb followed by an allophycocyanin (APC)-coupled anti-mouse IgG antibody.
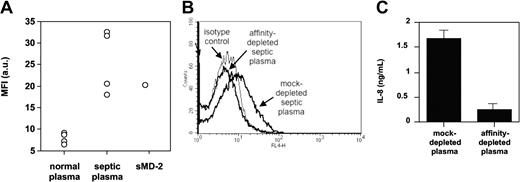
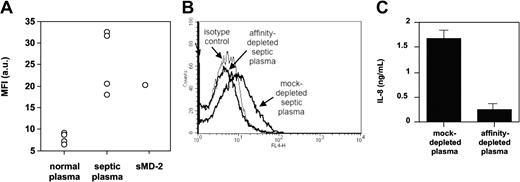
TLR4-HEK293 cells bound the anti-MD-2 18H10 mAb only when MD-2 was cotransfected or when exogenous soluble MD-2 was added to the cells (Figure 7). We also found that septic plasma, but not normal plasma, supported 18H10 mAb binding to TLR4-HEK293 cells by FACS, suggesting a transfer of soluble MD-2 contained in septic plasma to the TLR4-expressing cells (Figure 7). Interestingly, the addition of LPS, a classic TLR4/MD-2 ligand, did not modify the binding of soluble MD-2 to TLR4-HEK293 cells, as assessed by 18H10 mAb binding. We therefore took advantage of the binding of soluble MD-2 to TLR4 and used TLR4-HEK293 cells to affinity-deplete septic plasma from soluble MD-2. Septic plasma incubated with TLR4-HEK293 cells was successfully depleted of MD-2, as seen by 18H10 binding when reincubating depleted septic plasma with TLR4-HEK293 cells, whereas septic plasma incubated with untransfected HEK293 cells showed no MD-2 depletion (Figure 7). Importantly, affinity-depleted, but not mock-depleted, septic plasma lost its capacity to coactivate TLR4-HEK293 cells (Figure 7).
Anti-MD-2 18H10 mAb binding to TLR4-HEK293 cells. (A) FACS analysis of APC-conjugated anti-MD-2 18H10 mAb binding to TLR4-HEK293 cells in the presence of 10% normal plasma (n = 4), 10% septic plasma (n = 4), or recombinant soluble MD-2 (sMD-2). Mean fluorescence index indicates MFI; a.u., arbitrary units. (B) Loss of anti-MD-2 18H10 mAb binding to TLR4-HEK293 cells in the presence of septic plasma that has previously been affinity-depleted on TLR4-HEK293 cells, compared with septic plasma that has been mock-depleted on nontransfected HEK293 cells. This is 1 representative experiment from 3 similar experiments. (C) LPS activation of TLR4-HEK293 cells in the presence of 2% septic plasma that has been previously affinity-depleted on TLR4-HEK293 cells, compared with septic plasma that has been mock-depleted on nontransfected HEK293 cells. Mean ± 1 SD of triplicates. This is 1 representative experiment from 3 similar experiments.
Anti-MD-2 18H10 mAb binding to TLR4-HEK293 cells. (A) FACS analysis of APC-conjugated anti-MD-2 18H10 mAb binding to TLR4-HEK293 cells in the presence of 10% normal plasma (n = 4), 10% septic plasma (n = 4), or recombinant soluble MD-2 (sMD-2). Mean fluorescence index indicates MFI; a.u., arbitrary units. (B) Loss of anti-MD-2 18H10 mAb binding to TLR4-HEK293 cells in the presence of septic plasma that has previously been affinity-depleted on TLR4-HEK293 cells, compared with septic plasma that has been mock-depleted on nontransfected HEK293 cells. This is 1 representative experiment from 3 similar experiments. (C) LPS activation of TLR4-HEK293 cells in the presence of 2% septic plasma that has been previously affinity-depleted on TLR4-HEK293 cells, compared with septic plasma that has been mock-depleted on nontransfected HEK293 cells. Mean ± 1 SD of triplicates. This is 1 representative experiment from 3 similar experiments.
Effect of plasma on LPS binding to TLR4-expressing cells
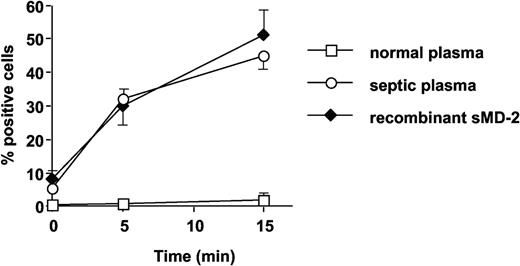
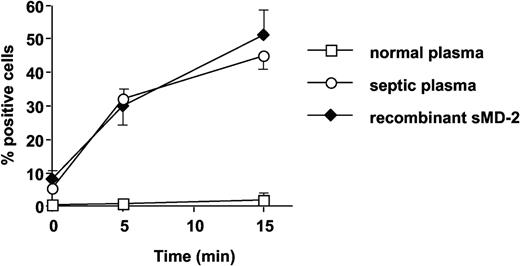
Septic plasma, but not normal plasma, supported FITC-conjugated LPS binding to TLR4-HEK293 cells, to levels very similar to those obtained with recombinant soluble MD-2 (Figure 8). The anti-MD-2 18H10 mAb did not interfere with septic plasma (sMD-2)-dependent LPS binding to TLR4 (data not shown).
Septic plasma and soluble MD-2, but not normal plasma, support FITC-LPS (1 μg/mL) binding to TLR4-HEK293 cells. Symbols represent septic plasma (○), soluble MD-2 (♦), and normal plasma (□). Mean ± 1 SD of triplicates. This is 1 representative experiment of 2 experiments. Similar results were obtained with 200 ng/mL FITC-LPS.
Septic plasma and soluble MD-2, but not normal plasma, support FITC-LPS (1 μg/mL) binding to TLR4-HEK293 cells. Symbols represent septic plasma (○), soluble MD-2 (♦), and normal plasma (□). Mean ± 1 SD of triplicates. This is 1 representative experiment of 2 experiments. Similar results were obtained with 200 ng/mL FITC-LPS.
Increased MD-2 mRNA in septic monocytes
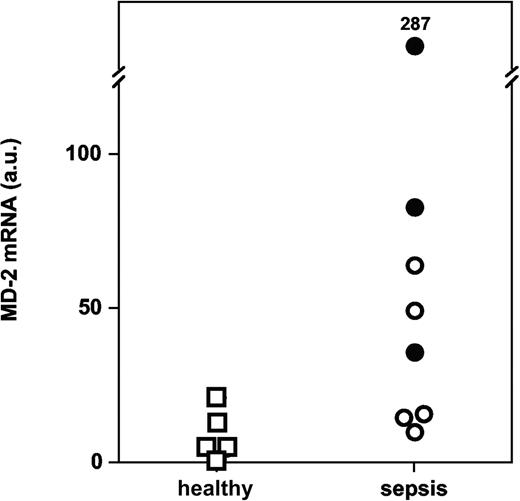
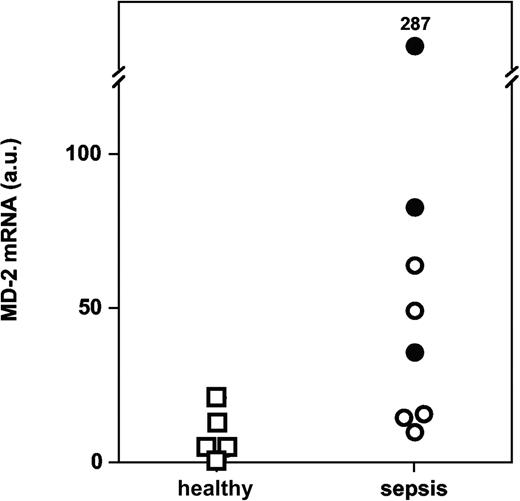
We performed quantitative PCR measurements of MD-2 mRNA in monocytes isolated from whole blood. We found significantly more MD-2 transcripts in monocytes from patients with severe sepsis and septic shock than in monocytes from volunteers (Figure 9; P = .019, Mann-Whitney U test).
MD-2 mRNA levels in monocytes from patients with severe sepsis and septic shock versus healthy volunteers (P = .019; arbitrary units, normalized for 18S ribosomal RNA). Symbols represent patients with severe sepsis (○) and septic shock (•) versus healthy volunteers (□).
MD-2 mRNA levels in monocytes from patients with severe sepsis and septic shock versus healthy volunteers (P = .019; arbitrary units, normalized for 18S ribosomal RNA). Symbols represent patients with severe sepsis (○) and septic shock (•) versus healthy volunteers (□).
Discussion
Herein, we show that plasma from patients with severe sepsis and septic shock, but not from volunteers, supports LPS activation of cells expressing TLR4 such as epithelial and endothelial cells. Sepsis and related complications, such as the acute respiratory distress syndrome (ARDS), are characterized by increased margination of circulating leukocytes in the microcirculation, procoagulant activity, and capillary leak of plasma proteins because of increased endothelial permeability.4 This results from broad endothelial cell activation, dysfunction, and ultimately endothelial cell death.4 Increased circulating levels of proinflammatory cytokines such as TNF-α and IL-1β were measured in plasma from patients with sepsis and ARDS.37-39 It is now clear that these circulating mediators are inactive because of an excess of natural inhibitors31,40,41 and can therefore hardly account for the observed endothelial activation. Soluble CD14 supports LPS activation of endothelial cells, an effect that is catalyzed by LBP.34,42 In the present work, we show that septic plasma is much more efficient than normal plasma in supporting LPS activation of primary human endothelial cells and that this is due to increased levels of the acute-phase proteins LBP and soluble CD14. In contrast with epithelial cells expressing only TLR4, endothelial cells express both TLR4 and MD-2 and do not require plasma-soluble MD-2 for LPS-mediated activation. If anything, elevated plasma levels of soluble MD-2 decrease LPS activation of endothelial cells.
In-organ inflammation is believed to play a role in the organ dysfunction observed in the severe forms of sepsis. An important proinflammatory activity has, for example, been measured in bronchoalveolar lavage fluids and in lung edema fluids from patients with ARDS accompanying sepsis.31,43,44 Epithelial cells are usually resistant to LPS, unless they express TLR4.8 In some circumstances, such as in the presence of interferon-γ, epithelial cells may up-regulate TLR4.8,9 However, the expression of TLR4 alone is not sufficient to generate a response to LPS. Soluble MD-2 was previously shown to support the LPS activation of TLR4-expressing cells.20 Our studies indicate that only plasma from patients with severe sepsis and septic shock, and not normal plasma, is able to mediate LPS activation of TLR4-expressing epithelial cells. Antibodies to LBP and CD14 blocked LPS activation of epithelial cells in the presence of septic plasma. However, the addition of the 2 plasma molecules in a recombinant form to normal plasma did not result in septic plasma-like effects. These results are consistent with a model in which LBP and soluble CD14 shuttle LPS to MD-2 in solution, which will in turn bring MD-2/LPS complexes to TLR4, as demonstrated by Kennedy et al.45 Importantly, Jia et al46 have recently shown that primary human lung epithelial cells expressed only TLR-4, but not MD-2. LPS responsiveness of these cells was only conferred by the presence of soluble MD-2 in solution or by transduction of these cells with MD-2 cDNA.46 The same differential expression of TLR4 and MD-2 could also be present in primary lung alveolar type II cells47 and in human intestinal epithelial cells from patients with inflammatory bowel disease.48 Interestingly, the same activity was found in the urine of a patient with gram-positive septic shock, possibly resulting from leakage of plasma proteins, including soluble MD-2, through a glomerular filter of increased permeability. MD-2 activity was also present in lung edema fluid from patients with ARDS. This activity was not present in lung edema fluid from critically ill patients intubated for cardiogenic shock. The origin of soluble MD-2 within the inflamed airways may be related to a local production by tissue or inflammatory cells or could come from septic plasma exudating through a leaky alveolar-capillary barrier. The plasma concentration found in the extravascular space during sepsis remains to be determined and may vary from organ to organ. It is, however, likely that protein concentrations in septic organs, despite a capillary leak, will be lower than those found in plasma. Supporting this, Heumann et al49 showed in an animal model of peritoneal inflammation that macrophages were surrounded by protein concentrations corresponding to approximately 1% of those measured in plasma.
In unpublished work, Kajikawa et al have shown that TLR4—but not MD-2—was significantly up-regulated by lung epithelial cells in a rabbit model of E coli pneumonia (Osamu Kajikawa and Thomas R. Martin, written personal communication, May 4, 2004). This is consistent with a model in which tissue epithelial cells only express TLR4 or alternatively can be stimulated to up-regulate TLR4, but not MD-2, by bacterial and inflammatory mediators. Even though these cells bear TLR4, they remain resistant to endotoxin. LPS responsiveness is conferred by soluble MD-2, produced either by neighboring cells or brought to the epithelial cells by plasma exudate. The formation of MD-2/LPS complexes is greatly enhanced by LBP and soluble CD14.45
Interestingly, the enhancing effect of septic plasma was not observed in myeloid cells. Rather, LPS induced less IL-8 and TNF-α in circulating leukocytes in the presence of septic plasma compared with normal plasma. We and others had previously shown that the monocyte-deactivating cytokine IL-10 present in septic plasma could account for the observed leukocyte LPS hyporesponsiveness.50,51 This effect was not observed in HEK293 cells expressing both surface TLR4 and MD-2, known for IL-10 resistance (J.P., unpublished observation, 2004). In these cells, both normal and septic plasma supported high levels of LPS activation, as if these cells did not need additional exogenous sources of soluble MD-2. High concentrations of septic plasma or recombinant soluble MD-2 dampened LPS responses of cells expressing both TLR4 and MD-2, confirming previous reports15 and raising the possibility that soluble MD-2 may play a down-regulating role in LPS-induced responses in the vascular compartment by binding LPS in solution and preventing it from interacting with membrane-bound TLR4/MD-2 complexes. Plasma factors other than IL-10 such as lipoproteins may well participate in the observed down-regulation of LPS-dependent leukocyte activation. In addition to LBP and soluble CD14,52 it is conceivable that plasma soluble MD-2 represents another “LPS-shuttling” molecule.
The putative presence of increased soluble MD-2 in plasma from patients with severe sepsis and septic shock raises the intriguing possibility that soluble MD-2 may act as an acute-phase reactant. We found that monocytes from patients with septic shock expressed increased levels of MD-2 mRNA as compared with monocytes from healthy subjects, which may point to a possible cellular source of circulating soluble MD-2. Interestingly, MD-2 mRNA levels were up-regulated in THP-1 cells by IL-6 and interferon-γ, typical inducers of acute-phase proteins.10 Furthermore, primary hepatocytes have been shown to express the MD-2 transcript.53 Additional studies on the MD-2 promoter,9 and on MD-2 expression in organs10 such as the liver from patients with sepsis, are needed to investigate this important point.
To address the specificity of the observed MD-2-like activity in septic plasma, we tested plasma from patients with acute exacerbation of rheumatoid arthritis, a classic condition with a noninfectious systemic acute-phase and inflammatory response. Rheumatoid arthritis plasma was much less potent in supporting LPS activation of TLR4-expressing epithelial cells than septic plasma, possibly because of a weaker acute-phase response as indicated by the mean C-reactive protein or because increased soluble MD-2 is specific for sepsis or infection.
Similar levels of soluble MD-2 activity were found in plasma from patients with gram-negative and gram-positive infection, as well as in those in which the pathogen could not be isolated. However, the soluble MD-2 activity seemed very specific for LPS and did not support gram-positive peptidoglycan activation of HepG2 cells expressing TLR1, TLR2, and TLR653 nor did it modify IL-1β activation of epithelial cells. It may be postulated that sepsis resulting from various bacterial infections triggers a soluble MD-2 plasma response that is only used by LPS, a situation reminiscent of the LBP acute-phase response. Importantly, plasma from patients who eventually died had a significantly higher soluble MD-2-like activity in plasma than survivors at the time of admission to the ICU. This “bioassay” may therefore be used as a prognostic test for a population of septic patients. Because of an overlap of values between survivors and nonsurvivors, it, however, cannot be used for a single patient. This also raises the possibility that high levels of plasma soluble MD-2 may negatively participate in the pathogenesis of septic shock.
Several lines of evidence indicate that circulating soluble MD-2 accounts for the activity measured in septic plasma. First, only the addition of recombinant soluble MD-2 could transform normal plasma into a sepsislike plasma with regard to LPS activation of TLR4-expressing cells. Second, MD-2 is secreted as multimers linked with disulfide bonds,24,26 which accounts for the high molecular weight (> 60 kDa) of the activity despite a predicted 20 to 25 kDa of the MD-2 monomer. Third, the activity could be precipitated with 50% ammonium sulfate, heat-inactivated, and was lost with the treatment of pronase, all properties fully compatible with the protein nature of MD-2 (J.P., S.S.V., and I.D.S., unpublished purification data, 2004). Fourth, experiments performed with a novel anti-MD-2 mAb which recognizes only TLR4-bound MD-2 indicated that (1) this antibody recognized TLR4-HEK293 cell surface only in the presence of septic plasma or after the addition of exogenous recombinant soluble MD-2; normal plasma did not support this effect; (2) affinity depletion of septic plasma using TLR4-HEK293 cells as an absorbent prevented anti-MD-2 binding to TLR4-expressing cells; and (3) depleted septic plasma lost its MD-2 activity in our bioassay. Fifth, identical physicochemical characteristics of the MD-2 activity were found in septic plasma, septic urine, and with recombinant soluble MD-2. The soluble MD-2 activity measured in these biologic fluids bound to a blue sepharose column and a cation exchange Mono S column and was found in the same elution fractions (J.P., S.S.V., and I.D.S., unpublished purification data, 2004). Finally, only septic plasma (by providing the necessary soluble MD-2 molecule) was able to support LPS binding to TLR4-expressing cells.
In conclusion, septic shock plasma supports significant endothelial and epithelial cell activation in the presence of LPS. Plasma exudation, by bringing soluble MD-2, allows TLR4-expressing epithelial cells to produce an inflammatory response to LPS, which is greatly enhanced by LBP and soluble CD14 cofactors. Endothelial cells express TLR4 and MD-2 and do not require additional soluble MD-2 to acquire LPS responsiveness. The septic plasma-enhancing effect is due to increased plasma levels of LBP and soluble CD14. In these latter cells and in leukocytes, which express both TLR4 and MD-2, LPS activation may be blunted by increased plasma levels of soluble MD-2. Leukocytes, but not endothelial cells, are further “deactivated” by circulating IL-10, which explains their poor response to LPS in the presence of septic plasma. This may be the basis for the concept that, at the same time during septic shock, proinflammatory, anti-inflammatory, and immunosuppressive responses can be observed, depending on the cell type studied.41,54 MD-2 may also represent an interesting future therapeutic target for septic shock.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2003-04-1290.
Supported by the Stanley Thomas Johnson Foundation (J.P.), by a grant from the National Institutes of Health (NIH; grant RO1 HL51856) (M.A.M.), and by research funding from NovImmune S.A. and the Swiss National Foundation for Research (grant 3200B0-105770) (J.P.).
Two of the authors (G.E. and B.D.) are employed by NovImmune S.A., Geneva, Switzerland, whose potential product, anti-MD-2 mAb (18H10), was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J.-M. Dayer, D. Trono, P. S. Tobias, K. Miyake, and M. Rothe for the gift of precious reagents. We also thank C. Gabay and T. Fumeaux for plasma samples and helpful discussions.